Professional Documents
Culture Documents
3.1 Crystal Fields: ?dti) (R)
3.1 Crystal Fields: ?dti) (R)
Uploaded by
Kamleshkekane1Copyright:
Available Formats
You might also like
- Questions 3rd Geologya and Ch. Applied 2023 PDFDocument20 pagesQuestions 3rd Geologya and Ch. Applied 2023 PDFAlaa KareemNo ratings yet
- Estonian Finnish Physics Olympiad 2012Document6 pagesEstonian Finnish Physics Olympiad 2012Science Olympiad Blog100% (2)
- CFT PDFDocument20 pagesCFT PDFRUFAS KANIKANTINo ratings yet
- Lecture 11 CFT 1Document4 pagesLecture 11 CFT 1ABDU11AH ShafiqNo ratings yet
- CFTDocument15 pagesCFTGaurav BothraNo ratings yet
- Effbcts of JH: t2g and Eg Lev-T2g Lobes Are Not Directed Towards The NearestDocument1 pageEffbcts of JH: t2g and Eg Lev-T2g Lobes Are Not Directed Towards The NearestSupriyaNo ratings yet
- Coord. Chem Notes - Modified 09-02-2023-1Document11 pagesCoord. Chem Notes - Modified 09-02-2023-1Ebsiba Beaula JNo ratings yet
- Lecture-12 CFT-1Document4 pagesLecture-12 CFT-1ABDU11AH ShafiqNo ratings yet
- 3.6 Jahn-Teller Effect: 2 High-Spin State (FigDocument1 page3.6 Jahn-Teller Effect: 2 High-Spin State (FigKamleshkekane1No ratings yet
- Oxidation States of Transition MetalsDocument7 pagesOxidation States of Transition MetalsMannevaram AbhinavareddiNo ratings yet
- Induced and Remanent MagnetismDocument14 pagesInduced and Remanent MagnetismArnaldo Hernández CardonaNo ratings yet
- Sliding On The Inside of A Conical Surface: R. L Opez-Ruiz and A. F. PachecoDocument26 pagesSliding On The Inside of A Conical Surface: R. L Opez-Ruiz and A. F. PachecoSubhankar HowladerNo ratings yet
- Crystal Field TheoryDocument26 pagesCrystal Field TheorySahil Qaiser100% (1)
- Gas Dynamics in Clusters of GalaxiesDocument30 pagesGas Dynamics in Clusters of GalaxiespjblkNo ratings yet
- VBTDocument40 pagesVBTLohith LoliNo ratings yet
- Photochemistry of Transition Metal Coordination Compounds: Cal Jfornia 90007Document28 pagesPhotochemistry of Transition Metal Coordination Compounds: Cal Jfornia 90007GopinathNo ratings yet
- InorganicDocument4 pagesInorganiccandy andersonNo ratings yet
- Figure 1. Arrangement of The Metal Ion (MDocument5 pagesFigure 1. Arrangement of The Metal Ion (MPhạm Gia KhánhNo ratings yet
- Astro160 Problems Solutions Philipsquestion2.4pg40Document66 pagesAstro160 Problems Solutions Philipsquestion2.4pg40Bobert100% (2)
- Coordin PDFDocument5 pagesCoordin PDFBhupinder AroraNo ratings yet
- Color of Transition Metal IonsDocument19 pagesColor of Transition Metal IonsSyedah Maira ShahNo ratings yet
- Lecture 2 CY101Document21 pagesLecture 2 CY101Gyanaranjan SahooNo ratings yet
- Answers To Assignment2-2009Document6 pagesAnswers To Assignment2-2009ElmIeyHakiemiEyNo ratings yet
- CFT 1Document19 pagesCFT 1Muhammad Umair IqbalNo ratings yet
- Ligand Field N MOTDocument12 pagesLigand Field N MOTLata Sharma100% (1)
- Ko Ordination S ChemieDocument31 pagesKo Ordination S ChemiePaoBenítezNo ratings yet
- Vasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3Document8 pagesVasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3SaqqerNo ratings yet
- Class 4Document13 pagesClass 4Muskan BiswalNo ratings yet
- Nature Paper On OrbitonsDocument4 pagesNature Paper On OrbitonsbasharatwantNo ratings yet
- Crystal Field Theory FDocument6 pagesCrystal Field Theory FAbdul WajidNo ratings yet
- Crystal Field Theory - NURDocument5 pagesCrystal Field Theory - NURNurhajrahNo ratings yet
- Double-Exchange Interaction and Orbital OrderingDocument32 pagesDouble-Exchange Interaction and Orbital OrderingDhanapal PalNo ratings yet
- Coordination NumberDocument11 pagesCoordination NumberSyed Qasim Shah100% (1)
- 3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Document1 page3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Kamleshkekane1No ratings yet
- Bonding Theories (CFT&LFT)Document54 pagesBonding Theories (CFT&LFT)delicakimmNo ratings yet
- 12 ChemDocument5 pages12 ChemBhoomi SinghNo ratings yet
- Chapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismDocument13 pagesChapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismAlia AliaNo ratings yet
- Crystal Field PotentialDocument3 pagesCrystal Field PotentialDubhe3No ratings yet
- Chapter04Document10 pagesChapter04aa9945158No ratings yet
- Thermal StressDocument10 pagesThermal Stresskannanmech87No ratings yet
- Chinese Physics Olympiad 2018 Finals Theoretical Exam: Translated By: Wai Ching Choi Edited By: Kushal ThamanDocument9 pagesChinese Physics Olympiad 2018 Finals Theoretical Exam: Translated By: Wai Ching Choi Edited By: Kushal ThamanPRITHVIRAJ GHOSHNo ratings yet
- Chapter 8 The D-And F-Block ElementsDocument30 pagesChapter 8 The D-And F-Block ElementsDharun SanthoshNo ratings yet
- Magnet 2Document12 pagesMagnet 2peoples1231697No ratings yet
- Crystal Field Theory (CFT)Document15 pagesCrystal Field Theory (CFT)veronicaNo ratings yet
- Water Waves Over Arrays of Horizontal Cylinders: Band Gaps and Bragg ResonanceDocument21 pagesWater Waves Over Arrays of Horizontal Cylinders: Band Gaps and Bragg Resonanceichi0727No ratings yet
- The Behavior of Metals at Optical FrequenciesDocument15 pagesThe Behavior of Metals at Optical Frequenciesjayaprakash2020No ratings yet
- Chapter 12 - Atoms-Saju-Hsslive PDFDocument9 pagesChapter 12 - Atoms-Saju-Hsslive PDFAmiNo ratings yet
- Lect 15Document13 pagesLect 15JK JKNo ratings yet
- J For A A: CodgumtionDocument1 pageJ For A A: CodgumtionKamleshkekane1No ratings yet
- Magnetic Moments: Lecture 4. CHEM1902 Coordination ChemistryDocument4 pagesMagnetic Moments: Lecture 4. CHEM1902 Coordination ChemistryAminah SaqibNo ratings yet
- Harry B. Gray and C. J. Ballhusa - Molecular Orbital Theory For Square Planar Metal ComplexesDocument6 pagesHarry B. Gray and C. J. Ballhusa - Molecular Orbital Theory For Square Planar Metal ComplexesNuansak3No ratings yet
- Exam 1 RepeatDocument7 pagesExam 1 RepeatBakhita MaryamNo ratings yet
- Theory of Metal OxidationDocument16 pagesTheory of Metal OxidationDyah Ayu DaratikaNo ratings yet
- Inorganic - 1Document270 pagesInorganic - 1Creative ThinkerNo ratings yet
- R RR Y Y Y: Phas2222 Quantum Physics Problem Sheet 4 (P2222.4) To Be Handed in by 5pm On Friday 7 December 2007Document1 pageR RR Y Y Y: Phas2222 Quantum Physics Problem Sheet 4 (P2222.4) To Be Handed in by 5pm On Friday 7 December 2007ShootingStarPhotonsNo ratings yet
- Introduction to Non-Linear Mechanics. (AM-11), Volume 11From EverandIntroduction to Non-Linear Mechanics. (AM-11), Volume 11No ratings yet
- Concepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1From EverandConcepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1No ratings yet
- The Enigmatic Electron: Electron Behaviour and How It Influences Our LivesFrom EverandThe Enigmatic Electron: Electron Behaviour and How It Influences Our LivesNo ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- It At: Hubbard ModelDocument1 pageIt At: Hubbard ModelKamleshkekane1No ratings yet
- # (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelDocument1 page# (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Jar, JZ,: Solutiom To The ProblemsDocument1 pageJar, JZ,: Solutiom To The ProblemsKamleshkekane1No ratings yet
- Mott Transition and Hubbard Model: Right: SchematicDocument1 pageMott Transition and Hubbard Model: Right: SchematicKamleshkekane1No ratings yet
- Hubbard: and ModelDocument1 pageHubbard: and ModelKamleshkekane1No ratings yet
- Hubbard Model: ModelsDocument1 pageHubbard Model: ModelsKamleshkekane1No ratings yet
- And Hubbard Model: To (FS)Document1 pageAnd Hubbard Model: To (FS)Kamleshkekane1No ratings yet
- 4.2 Mott Damition: Na 4a. CriticalDocument1 page4.2 Mott Damition: Na 4a. CriticalKamleshkekane1No ratings yet
- Mott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionDocument1 pageMott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionKamleshkekane1No ratings yet
- A3s At: Mott Transition and Hubbard ModelDocument1 pageA3s At: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Crystal Field Theory: in IsDocument1 pageCrystal Field Theory: in IsKamleshkekane1No ratings yet
- Mott Llansition: Is IsDocument1 pageMott Llansition: Is IsKamleshkekane1No ratings yet
- It Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityDocument1 pageIt Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityKamleshkekane1No ratings yet
- 144 3 Crystal: H Ma3h"Document1 page144 3 Crystal: H Ma3h"Kamleshkekane1No ratings yet
- Transition: Is COO A ofDocument1 pageTransition: Is COO A ofKamleshkekane1No ratings yet
- Solutions To The Problems: FreeDocument1 pageSolutions To The Problems: FreeKamleshkekane1No ratings yet
- Solutions To The Problems 143: H,, 34.2 TeslaDocument1 pageSolutions To The Problems 143: H,, 34.2 TeslaKamleshkekane1No ratings yet
- Crystal: Field TheoryDocument1 pageCrystal: Field TheoryKamleshkekane1No ratings yet
- A $ (BLT) A: Crystal Field TheoryDocument1 pageA $ (BLT) A: Crystal Field TheoryKamleshkekane1No ratings yet
- t2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryDocument1 paget2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryKamleshkekane1No ratings yet
- @Q/&GFQGZ.: Solutions To The ProblemsDocument1 page@Q/&GFQGZ.: Solutions To The ProblemsKamleshkekane1No ratings yet
- Field: Ch. 3 Crystal TheoryDocument1 pageField: Ch. 3 Crystal TheoryKamleshkekane1No ratings yet
- Solutions The: So Are ToDocument1 pageSolutions The: So Are ToKamleshkekane1No ratings yet
3.1 Crystal Fields: ?dti) (R)
3.1 Crystal Fields: ?dti) (R)
Uploaded by
Kamleshkekane1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
3.1 Crystal Fields: ?dti) (R)
3.1 Crystal Fields: ?dti) (R)
Uploaded by
Kamleshkekane1Copyright:
Available Formats
3.
1 Crystal Fields 77
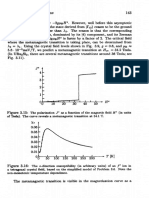
Figure 3.1: Left: Schematic representation of the local environment of transition
metal ions in the undistorted perovskite structure, and in related structures such as
the KzNiF4 structure. In LaTiOJ, a Ti ion (filled circle) is sitting in the octahedral
environment of surrounding oxygen ions (empty circles); in LazCuOc, the black circle
is a Cu ion. Right: the horizontal plane through the octahedron centers is the
characteristic Cu02 plane of the cuprates.
what is the ground state of the 3d1 configuration? If the Ti3+ ion were
alone in vacuum, and if we neglected the spin-orbit coupling (which is
indeed small for Ti), the electron could be in any of the five 3d orbital
states, and its ground state would be tenfold degenerate (counting also
the spin degeneracy). If we included the spin-orbit interaction, Hund's
third rule would tell us that the ground state is the fourfold degenerate
J = 3/2 level.
The essential point, though, is that the motion of the d-electron is
governed not only by the potential of the Ti3+ ion core, which we include
in the ionic Hamiltonian ?ifTi), but also by the electrostatic potential
of the surrounding six oxygen ions
6
3t3d(r) = ?dTi)(r) + V(O"Y)(r- Rj).
j=1
The very notion of a 3d-state derives from the eigenvalue problem
of the spherically symmetrical 3t(Ti).If the anisotropic field produced
by the oxygen ions were very strong, we should start the solution of
the Schrodinger equation from scratch. Fortunately, it is an acceptable
You might also like
- Questions 3rd Geologya and Ch. Applied 2023 PDFDocument20 pagesQuestions 3rd Geologya and Ch. Applied 2023 PDFAlaa KareemNo ratings yet
- Estonian Finnish Physics Olympiad 2012Document6 pagesEstonian Finnish Physics Olympiad 2012Science Olympiad Blog100% (2)
- CFT PDFDocument20 pagesCFT PDFRUFAS KANIKANTINo ratings yet
- Lecture 11 CFT 1Document4 pagesLecture 11 CFT 1ABDU11AH ShafiqNo ratings yet
- CFTDocument15 pagesCFTGaurav BothraNo ratings yet
- Effbcts of JH: t2g and Eg Lev-T2g Lobes Are Not Directed Towards The NearestDocument1 pageEffbcts of JH: t2g and Eg Lev-T2g Lobes Are Not Directed Towards The NearestSupriyaNo ratings yet
- Coord. Chem Notes - Modified 09-02-2023-1Document11 pagesCoord. Chem Notes - Modified 09-02-2023-1Ebsiba Beaula JNo ratings yet
- Lecture-12 CFT-1Document4 pagesLecture-12 CFT-1ABDU11AH ShafiqNo ratings yet
- 3.6 Jahn-Teller Effect: 2 High-Spin State (FigDocument1 page3.6 Jahn-Teller Effect: 2 High-Spin State (FigKamleshkekane1No ratings yet
- Oxidation States of Transition MetalsDocument7 pagesOxidation States of Transition MetalsMannevaram AbhinavareddiNo ratings yet
- Induced and Remanent MagnetismDocument14 pagesInduced and Remanent MagnetismArnaldo Hernández CardonaNo ratings yet
- Sliding On The Inside of A Conical Surface: R. L Opez-Ruiz and A. F. PachecoDocument26 pagesSliding On The Inside of A Conical Surface: R. L Opez-Ruiz and A. F. PachecoSubhankar HowladerNo ratings yet
- Crystal Field TheoryDocument26 pagesCrystal Field TheorySahil Qaiser100% (1)
- Gas Dynamics in Clusters of GalaxiesDocument30 pagesGas Dynamics in Clusters of GalaxiespjblkNo ratings yet
- VBTDocument40 pagesVBTLohith LoliNo ratings yet
- Photochemistry of Transition Metal Coordination Compounds: Cal Jfornia 90007Document28 pagesPhotochemistry of Transition Metal Coordination Compounds: Cal Jfornia 90007GopinathNo ratings yet
- InorganicDocument4 pagesInorganiccandy andersonNo ratings yet
- Figure 1. Arrangement of The Metal Ion (MDocument5 pagesFigure 1. Arrangement of The Metal Ion (MPhạm Gia KhánhNo ratings yet
- Astro160 Problems Solutions Philipsquestion2.4pg40Document66 pagesAstro160 Problems Solutions Philipsquestion2.4pg40Bobert100% (2)
- Coordin PDFDocument5 pagesCoordin PDFBhupinder AroraNo ratings yet
- Color of Transition Metal IonsDocument19 pagesColor of Transition Metal IonsSyedah Maira ShahNo ratings yet
- Lecture 2 CY101Document21 pagesLecture 2 CY101Gyanaranjan SahooNo ratings yet
- Answers To Assignment2-2009Document6 pagesAnswers To Assignment2-2009ElmIeyHakiemiEyNo ratings yet
- CFT 1Document19 pagesCFT 1Muhammad Umair IqbalNo ratings yet
- Ligand Field N MOTDocument12 pagesLigand Field N MOTLata Sharma100% (1)
- Ko Ordination S ChemieDocument31 pagesKo Ordination S ChemiePaoBenítezNo ratings yet
- Vasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3Document8 pagesVasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3SaqqerNo ratings yet
- Class 4Document13 pagesClass 4Muskan BiswalNo ratings yet
- Nature Paper On OrbitonsDocument4 pagesNature Paper On OrbitonsbasharatwantNo ratings yet
- Crystal Field Theory FDocument6 pagesCrystal Field Theory FAbdul WajidNo ratings yet
- Crystal Field Theory - NURDocument5 pagesCrystal Field Theory - NURNurhajrahNo ratings yet
- Double-Exchange Interaction and Orbital OrderingDocument32 pagesDouble-Exchange Interaction and Orbital OrderingDhanapal PalNo ratings yet
- Coordination NumberDocument11 pagesCoordination NumberSyed Qasim Shah100% (1)
- 3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Document1 page3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Kamleshkekane1No ratings yet
- Bonding Theories (CFT&LFT)Document54 pagesBonding Theories (CFT&LFT)delicakimmNo ratings yet
- 12 ChemDocument5 pages12 ChemBhoomi SinghNo ratings yet
- Chapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismDocument13 pagesChapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismAlia AliaNo ratings yet
- Crystal Field PotentialDocument3 pagesCrystal Field PotentialDubhe3No ratings yet
- Chapter04Document10 pagesChapter04aa9945158No ratings yet
- Thermal StressDocument10 pagesThermal Stresskannanmech87No ratings yet
- Chinese Physics Olympiad 2018 Finals Theoretical Exam: Translated By: Wai Ching Choi Edited By: Kushal ThamanDocument9 pagesChinese Physics Olympiad 2018 Finals Theoretical Exam: Translated By: Wai Ching Choi Edited By: Kushal ThamanPRITHVIRAJ GHOSHNo ratings yet
- Chapter 8 The D-And F-Block ElementsDocument30 pagesChapter 8 The D-And F-Block ElementsDharun SanthoshNo ratings yet
- Magnet 2Document12 pagesMagnet 2peoples1231697No ratings yet
- Crystal Field Theory (CFT)Document15 pagesCrystal Field Theory (CFT)veronicaNo ratings yet
- Water Waves Over Arrays of Horizontal Cylinders: Band Gaps and Bragg ResonanceDocument21 pagesWater Waves Over Arrays of Horizontal Cylinders: Band Gaps and Bragg Resonanceichi0727No ratings yet
- The Behavior of Metals at Optical FrequenciesDocument15 pagesThe Behavior of Metals at Optical Frequenciesjayaprakash2020No ratings yet
- Chapter 12 - Atoms-Saju-Hsslive PDFDocument9 pagesChapter 12 - Atoms-Saju-Hsslive PDFAmiNo ratings yet
- Lect 15Document13 pagesLect 15JK JKNo ratings yet
- J For A A: CodgumtionDocument1 pageJ For A A: CodgumtionKamleshkekane1No ratings yet
- Magnetic Moments: Lecture 4. CHEM1902 Coordination ChemistryDocument4 pagesMagnetic Moments: Lecture 4. CHEM1902 Coordination ChemistryAminah SaqibNo ratings yet
- Harry B. Gray and C. J. Ballhusa - Molecular Orbital Theory For Square Planar Metal ComplexesDocument6 pagesHarry B. Gray and C. J. Ballhusa - Molecular Orbital Theory For Square Planar Metal ComplexesNuansak3No ratings yet
- Exam 1 RepeatDocument7 pagesExam 1 RepeatBakhita MaryamNo ratings yet
- Theory of Metal OxidationDocument16 pagesTheory of Metal OxidationDyah Ayu DaratikaNo ratings yet
- Inorganic - 1Document270 pagesInorganic - 1Creative ThinkerNo ratings yet
- R RR Y Y Y: Phas2222 Quantum Physics Problem Sheet 4 (P2222.4) To Be Handed in by 5pm On Friday 7 December 2007Document1 pageR RR Y Y Y: Phas2222 Quantum Physics Problem Sheet 4 (P2222.4) To Be Handed in by 5pm On Friday 7 December 2007ShootingStarPhotonsNo ratings yet
- Introduction to Non-Linear Mechanics. (AM-11), Volume 11From EverandIntroduction to Non-Linear Mechanics. (AM-11), Volume 11No ratings yet
- Concepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1From EverandConcepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1No ratings yet
- The Enigmatic Electron: Electron Behaviour and How It Influences Our LivesFrom EverandThe Enigmatic Electron: Electron Behaviour and How It Influences Our LivesNo ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- It At: Hubbard ModelDocument1 pageIt At: Hubbard ModelKamleshkekane1No ratings yet
- # (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelDocument1 page# (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Jar, JZ,: Solutiom To The ProblemsDocument1 pageJar, JZ,: Solutiom To The ProblemsKamleshkekane1No ratings yet
- Mott Transition and Hubbard Model: Right: SchematicDocument1 pageMott Transition and Hubbard Model: Right: SchematicKamleshkekane1No ratings yet
- Hubbard: and ModelDocument1 pageHubbard: and ModelKamleshkekane1No ratings yet
- Hubbard Model: ModelsDocument1 pageHubbard Model: ModelsKamleshkekane1No ratings yet
- And Hubbard Model: To (FS)Document1 pageAnd Hubbard Model: To (FS)Kamleshkekane1No ratings yet
- 4.2 Mott Damition: Na 4a. CriticalDocument1 page4.2 Mott Damition: Na 4a. CriticalKamleshkekane1No ratings yet
- Mott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionDocument1 pageMott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionKamleshkekane1No ratings yet
- A3s At: Mott Transition and Hubbard ModelDocument1 pageA3s At: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Crystal Field Theory: in IsDocument1 pageCrystal Field Theory: in IsKamleshkekane1No ratings yet
- Mott Llansition: Is IsDocument1 pageMott Llansition: Is IsKamleshkekane1No ratings yet
- It Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityDocument1 pageIt Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityKamleshkekane1No ratings yet
- 144 3 Crystal: H Ma3h"Document1 page144 3 Crystal: H Ma3h"Kamleshkekane1No ratings yet
- Transition: Is COO A ofDocument1 pageTransition: Is COO A ofKamleshkekane1No ratings yet
- Solutions To The Problems: FreeDocument1 pageSolutions To The Problems: FreeKamleshkekane1No ratings yet
- Solutions To The Problems 143: H,, 34.2 TeslaDocument1 pageSolutions To The Problems 143: H,, 34.2 TeslaKamleshkekane1No ratings yet
- Crystal: Field TheoryDocument1 pageCrystal: Field TheoryKamleshkekane1No ratings yet
- A $ (BLT) A: Crystal Field TheoryDocument1 pageA $ (BLT) A: Crystal Field TheoryKamleshkekane1No ratings yet
- t2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryDocument1 paget2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryKamleshkekane1No ratings yet
- @Q/&GFQGZ.: Solutions To The ProblemsDocument1 page@Q/&GFQGZ.: Solutions To The ProblemsKamleshkekane1No ratings yet
- Field: Ch. 3 Crystal TheoryDocument1 pageField: Ch. 3 Crystal TheoryKamleshkekane1No ratings yet
- Solutions The: So Are ToDocument1 pageSolutions The: So Are ToKamleshkekane1No ratings yet