Professional Documents
Culture Documents
3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1
3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1
Uploaded by
Kamleshkekane10 ratings0% found this document useful (0 votes)
12 views1 pageThis document discusses crystal field effects on transition metal ions. It explains that crystal fields have a noticeable effect on 3d electrons in transition metals, but a much weaker effect on 4f electrons in rare earth metals. For rare earth metals, the order of relevant interactions is exchange splittings > spin-orbit coupling > crystal fields. For transition metals like Cu2+, the order is exchange splittings > crystal fields > spin-orbit coupling, meaning crystal fields dominate over spin-orbit coupling and Hund's third rule no longer applies. Crystal fields mix states within a given term for transition metals.
Original Description:
General Document 41
Original Title
00095___7445b16af08a60bc35dbac66f6fb214b
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses crystal field effects on transition metal ions. It explains that crystal fields have a noticeable effect on 3d electrons in transition metals, but a much weaker effect on 4f electrons in rare earth metals. For rare earth metals, the order of relevant interactions is exchange splittings > spin-orbit coupling > crystal fields. For transition metals like Cu2+, the order is exchange splittings > crystal fields > spin-orbit coupling, meaning crystal fields dominate over spin-orbit coupling and Hund's third rule no longer applies. Crystal fields mix states within a given term for transition metals.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
12 views1 page3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1
3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1
Uploaded by
Kamleshkekane1This document discusses crystal field effects on transition metal ions. It explains that crystal fields have a noticeable effect on 3d electrons in transition metals, but a much weaker effect on 4f electrons in rare earth metals. For rare earth metals, the order of relevant interactions is exchange splittings > spin-orbit coupling > crystal fields. For transition metals like Cu2+, the order is exchange splittings > crystal fields > spin-orbit coupling, meaning crystal fields dominate over spin-orbit coupling and Hund's third rule no longer applies. Crystal fields mix states within a given term for transition metals.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
3.
1 Crystal Fields 79
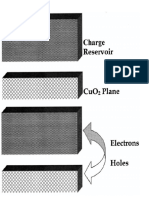
called K2NiF4 structure [84]which differs from the perovskite structure
by having two A 0 planes (rather than one A 0 plane) in between two
consecutive BO2 planes. The CuOz plane of LaZCu04 (shown in the
right panel of Fig. 3.1) is a famous object because it is thought to be
the relevant structural unit of the high-?’, cuprates, Thus the local
environment of the Cu ions is approximately octahedral as in Fig. 3.1.
Identifying the valence states as LgsCu2+Oi-, we immediately see that
the only “interesting” ion (i.e., one with a partially filled shell) is Cu2+
which has the configuration 3 8 . Since 3d1’ would be a completely filled
shell, it is justified to consider Cu2+ as having one d-hole. The problem
of the electronic structure of Cu2+ is thus the mirror image of that of
the structure of Ti3+: there we were interested in the only d-state which
is occupied; here in the only state which is unoccupied.
Crystal field effects will also give an explanation of the missing or-
bital contribution to the susceptibility of transition metal ions. This
raises, however, another question: why have crystal fields seemingly no
effect on .Qf-electrons? In fact they do have an effect, only a much
weaker one than on 3d-electrons. The reason is that the 4f orbitals
are lying so deep within the ion core that other occupied shells of the
same ion largely screen out the potential of the surrounding ions. 4f
electrons indeed hardly notice whether they are in an ion embedded in
a crystal, or in a free ion. The 4f electrons in rare-earth-based solids
give an example of a weak crystal field satisfying
exchange splittings > spin-orbit coupling > crystal fields
Because of this order of the relevant coupling strengths, even Hund’s
third rule takes precedence over lattice effects. Crystal field effects (and
quite subtle ones at that!) act only within a given J-manifold.
Transition metal ions (particularly 3d-ions) belong to the category
of intermediate crystal field strengths
exchange splittings > crystal fields > spin-orbit coupling
Since crystal fields now dominate over the spin-orbit coupling, Hund’s
third rule ceases to apply. This means that though L and S remain valid
quantum numbers, and their values are still given by Hund’s first and
second rule, J is no longer a good quantum number. An intermediate
crystal field mixes states within a given ( L ,S) term.
You might also like
- 11-Kinematics Practice Test PDFDocument2 pages11-Kinematics Practice Test PDFD'ferti Anggraeni0% (2)
- Sales Agent Form PDFDocument1 pageSales Agent Form PDFJaafar SorianoNo ratings yet
- X-RAY DIFFRACTION and Crystal DefectsDocument68 pagesX-RAY DIFFRACTION and Crystal DefectsAnjan Prasad100% (1)
- HSE Information Sheet Ageing Semi-Submersible Installations Offshore Information Sheet No 5 2007Document5 pagesHSE Information Sheet Ageing Semi-Submersible Installations Offshore Information Sheet No 5 2007Saeed JabbariNo ratings yet
- The D and F Block ElementsDocument16 pagesThe D and F Block Elementssyedasifbasha1990No ratings yet
- CFT 1Document19 pagesCFT 1Muhammad Umair IqbalNo ratings yet
- Ncert Sol D&FDocument16 pagesNcert Sol D&FKAVERI JAINNo ratings yet
- CHM 221 Lecture Note 1 - Transition ElementsDocument9 pagesCHM 221 Lecture Note 1 - Transition ElementsOlanrewaju Omowunmi GraceNo ratings yet
- CFT PDFDocument20 pagesCFT PDFRUFAS KANIKANTINo ratings yet
- Transition Metal 4Document4 pagesTransition Metal 4Sushant ShahNo ratings yet
- LanthanidesDocument2 pagesLanthanideswardaNo ratings yet
- 3 Field: FieldsDocument1 page3 Field: FieldsKamleshkekane1No ratings yet
- Lesson 4Document22 pagesLesson 4vadob71936No ratings yet
- 12 Chemistry Imp Ch8 5Document23 pages12 Chemistry Imp Ch8 5Ishant SahuNo ratings yet
- Transition Metal ChemistryDocument34 pagesTransition Metal ChemistryCowboy XxNo ratings yet
- D Block ElementsDocument36 pagesD Block ElementsMagistrina PrimaNo ratings yet
- OrbitalsDocument1 pageOrbitalshttp://spmchem.blogspot.com/No ratings yet
- Topic 4 BondingDocument29 pagesTopic 4 BondingXandi NalepaNo ratings yet
- Chapter 4 ChemistryDocument4 pagesChapter 4 Chemistryvasutiwari3037x2102No ratings yet
- The Alkali AtomsDocument8 pagesThe Alkali AtomsNur IzzatiNo ratings yet
- Crystal Field Theory - NURDocument5 pagesCrystal Field Theory - NURNurhajrahNo ratings yet
- Bonding Theories (CFT&LFT)Document54 pagesBonding Theories (CFT&LFT)delicakimmNo ratings yet
- D Block Imp QuestionsDocument4 pagesD Block Imp QuestionsAnanya SrivastavaNo ratings yet
- Contentpage Tafssp 157 17Document77 pagesContentpage Tafssp 157 17prabs20069178No ratings yet
- 8.6 Spinel, Perovskite, and Rutile Structures PDFDocument5 pages8.6 Spinel, Perovskite, and Rutile Structures PDFΑντώνης ΜακρίδηςNo ratings yet
- Basic Semiconductor PhysicsDocument311 pagesBasic Semiconductor PhysicseeshgargNo ratings yet
- CHM 221 Lecture Note 2022-2023Document18 pagesCHM 221 Lecture Note 2022-2023Olanrewaju Omowunmi GraceNo ratings yet
- Class 12 Study Material Chemistry SA-1Document92 pagesClass 12 Study Material Chemistry SA-1VipinVKumarNo ratings yet
- Oxidation States of Transition MetalsDocument5 pagesOxidation States of Transition MetalskushanNo ratings yet
- Che 91164 RevisionDocument0 pagesChe 91164 Revisionapi-218511741No ratings yet
- Chemical Bonding NotesDocument9 pagesChemical Bonding NotesMohammed YusufNo ratings yet
- Chemistry The D and F Block Elements Q&A 5marksDocument14 pagesChemistry The D and F Block Elements Q&A 5marksPramit RanjanNo ratings yet
- Doublet Sate of Alkali AtomDocument6 pagesDoublet Sate of Alkali AtomNur IzzatiNo ratings yet
- D and F Block Worksheet 2Document8 pagesD and F Block Worksheet 2Aryan JainNo ratings yet
- D and F Block ElementsDocument8 pagesD and F Block ElementsPrashanth SNo ratings yet
- Transition Metal ChemistryDocument36 pagesTransition Metal ChemistryRojo JohnNo ratings yet
- J For A A: CodgumtionDocument1 pageJ For A A: CodgumtionKamleshkekane1No ratings yet
- 1 Crystal Defects - Ch04Document72 pages1 Crystal Defects - Ch04Kimberly Joy FerrerNo ratings yet
- MOT NewDocument37 pagesMOT Newdsw27No ratings yet
- Classnotes 10Document6 pagesClassnotes 10Muhammd Usman MalikNo ratings yet
- 0953-4075 33 2 310Document15 pages0953-4075 33 2 310beaveacedemiaNo ratings yet
- Ligand Field StrengthDocument24 pagesLigand Field StrengthIrvandar NurviandyNo ratings yet
- Class 12 CH 8 D and F Block ElementsDocument5 pagesClass 12 CH 8 D and F Block ElementsKumar Pratik50% (2)
- ElectronDocument24 pagesElectronKC BakiaoNo ratings yet
- 3.1 Crystal Fields: ?dti) (R)Document1 page3.1 Crystal Fields: ?dti) (R)Kamleshkekane1No ratings yet
- Crystal Field TheoryDocument7 pagesCrystal Field TheoryD GNo ratings yet
- Orbital Physics in Transition-Metal Oxides From First-PrinciplesDocument8 pagesOrbital Physics in Transition-Metal Oxides From First-PrinciplesalfonsoNo ratings yet
- D Block (2012 13)Document8 pagesD Block (2012 13)Anonymous 8VJhV1eI2y100% (1)
- Tid/g: P-D Hybridization Gives Rise To An Antiferromagnetic Cu2+-O-exchangeDocument1 pageTid/g: P-D Hybridization Gives Rise To An Antiferromagnetic Cu2+-O-exchangeKetanNo ratings yet
- Chap 7Document6 pagesChap 7api-3704690No ratings yet
- D & F Block Elements NCERTDocument18 pagesD & F Block Elements NCERTmehakNo ratings yet
- MTRLDocument35 pagesMTRLVictor Anthony CuaresmaNo ratings yet
- Transition Metal ChemistryDocument33 pagesTransition Metal ChemistrySilas KipkogeiNo ratings yet
- No. of Atom, Packing Fraction, Co-Ordination Number - 08.6.22Document47 pagesNo. of Atom, Packing Fraction, Co-Ordination Number - 08.6.22mithunesh 07No ratings yet
- Solid and Semiconductor 2021Document18 pagesSolid and Semiconductor 2021Yaghya SoniNo ratings yet
- Holes in A Two-Dimensional Quantum AntiferromagnetDocument38 pagesHoles in A Two-Dimensional Quantum AntiferromagnetsantoshkudNo ratings yet
- Crystal Structures: Face Center CubicDocument8 pagesCrystal Structures: Face Center CubicJoyal PeterNo ratings yet
- Forces of AttractionDocument30 pagesForces of AttractionDiamonette SynconNo ratings yet
- Electronic Layers in Copper Oxide SuperconductorsDocument1 pageElectronic Layers in Copper Oxide SuperconductorsSJNo ratings yet
- HybridizationDocument9 pagesHybridizationSatyaki MajumdarNo ratings yet
- Progress in the Science and Technology of the Rare Earths: Volume 2From EverandProgress in the Science and Technology of the Rare Earths: Volume 2No ratings yet
- Festkörper Probleme: Plenary Lectures of the Divisions Semiconductor Physics, Surface Physics, Low Temperature Physics, High Polymers, Thermodynamics and Statistical Mechanics, of the German Physical Society, Münster, March 19–24, 1973From EverandFestkörper Probleme: Plenary Lectures of the Divisions Semiconductor Physics, Surface Physics, Low Temperature Physics, High Polymers, Thermodynamics and Statistical Mechanics, of the German Physical Society, Münster, March 19–24, 1973No ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- Hubbard Model: ModelsDocument1 pageHubbard Model: ModelsKamleshkekane1No ratings yet
- It At: Hubbard ModelDocument1 pageIt At: Hubbard ModelKamleshkekane1No ratings yet
- # (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelDocument1 page# (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- 4.2 Mott Damition: Na 4a. CriticalDocument1 page4.2 Mott Damition: Na 4a. CriticalKamleshkekane1No ratings yet
- And Hubbard Model: To (FS)Document1 pageAnd Hubbard Model: To (FS)Kamleshkekane1No ratings yet
- Transition: Is COO A ofDocument1 pageTransition: Is COO A ofKamleshkekane1No ratings yet
- Mott Transition and Hubbard Model: Right: SchematicDocument1 pageMott Transition and Hubbard Model: Right: SchematicKamleshkekane1No ratings yet
- Hubbard: and ModelDocument1 pageHubbard: and ModelKamleshkekane1No ratings yet
- A3s At: Mott Transition and Hubbard ModelDocument1 pageA3s At: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Jar, JZ,: Solutiom To The ProblemsDocument1 pageJar, JZ,: Solutiom To The ProblemsKamleshkekane1No ratings yet
- It Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityDocument1 pageIt Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityKamleshkekane1No ratings yet
- Mott Llansition: Is IsDocument1 pageMott Llansition: Is IsKamleshkekane1No ratings yet
- 144 3 Crystal: H Ma3h"Document1 page144 3 Crystal: H Ma3h"Kamleshkekane1No ratings yet
- Solutions To The Problems 143: H,, 34.2 TeslaDocument1 pageSolutions To The Problems 143: H,, 34.2 TeslaKamleshkekane1No ratings yet
- Mott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionDocument1 pageMott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionKamleshkekane1No ratings yet
- Solutions To The Problems: FreeDocument1 pageSolutions To The Problems: FreeKamleshkekane1No ratings yet
- Crystal Field Theory: in IsDocument1 pageCrystal Field Theory: in IsKamleshkekane1No ratings yet
- A $ (BLT) A: Crystal Field TheoryDocument1 pageA $ (BLT) A: Crystal Field TheoryKamleshkekane1No ratings yet
- Crystal: Field TheoryDocument1 pageCrystal: Field TheoryKamleshkekane1No ratings yet
- @Q/&GFQGZ.: Solutions To The ProblemsDocument1 page@Q/&GFQGZ.: Solutions To The ProblemsKamleshkekane1No ratings yet
- Field: Ch. 3 Crystal TheoryDocument1 pageField: Ch. 3 Crystal TheoryKamleshkekane1No ratings yet
- t2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryDocument1 paget2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryKamleshkekane1No ratings yet
- Solutions The: So Are ToDocument1 pageSolutions The: So Are ToKamleshkekane1No ratings yet
- MicroControllers HandbookDocument13 pagesMicroControllers Handbookjhon doeNo ratings yet
- Solution TestDocument3 pagesSolution TestmridulNo ratings yet
- FabFilter Volcano 2Document43 pagesFabFilter Volcano 2David Esteves RuizNo ratings yet
- CV - Emad Jamshidi: P Date of Birth Gender Citizenship E-Mail Phone Work Mobile Telephone Current ProfessionDocument1 pageCV - Emad Jamshidi: P Date of Birth Gender Citizenship E-Mail Phone Work Mobile Telephone Current ProfessionEmad JamshidiNo ratings yet
- Profitability Analysis of Chilime Hydropower CompanyDocument10 pagesProfitability Analysis of Chilime Hydropower CompanySangita GhimireNo ratings yet
- Tips On UpcatDocument5 pagesTips On UpcatAira Mae AloverosNo ratings yet
- CPP QN 2019Document16 pagesCPP QN 2019kanishk vohraNo ratings yet
- Load at Yield Point (N) Yield Strength (Mpa) Maximum Load (N) Ultimater Strength (Mpa)Document4 pagesLoad at Yield Point (N) Yield Strength (Mpa) Maximum Load (N) Ultimater Strength (Mpa)Anonymous SJE1kG5AkNNo ratings yet
- Current Stock ListDocument8 pagesCurrent Stock ListOjhal RaiNo ratings yet
- Problems in DecibelsDocument6 pagesProblems in DecibelsLara Jane ReyesNo ratings yet
- Us0ab 21023133 21023133Document1 pageUs0ab 21023133 21023133krish.gkavarapuNo ratings yet
- Water Management Rules As MWELO in Los AngelesDocument2 pagesWater Management Rules As MWELO in Los AngelesPrecise Landscape Water ConservationNo ratings yet
- Team6 Legend 5BVLCDocument8 pagesTeam6 Legend 5BVLCAna Sofia MuñizNo ratings yet
- Lecture Notes Consumer Behavior TextbookDocument185 pagesLecture Notes Consumer Behavior TextbookSao Nguyễn ToànNo ratings yet
- Carlyn Shear ResumeDocument1 pageCarlyn Shear ResumecarlynshearNo ratings yet
- Wiii WaddDocument6 pagesWiii WaddMuhammad Daffa A SNo ratings yet
- En Solutions-Brochure Axiocam FamilyDocument42 pagesEn Solutions-Brochure Axiocam FamilyLara MatosNo ratings yet
- Landscape and Urban Planning: Jie Su, Alexandros GasparatosDocument15 pagesLandscape and Urban Planning: Jie Su, Alexandros GasparatosMaria Claudia VergaraNo ratings yet
- Activity 3 EntrepDocument2 pagesActivity 3 EntrepCHLOE ANNE CORDIALNo ratings yet
- MOST 3rd RevisionDocument654 pagesMOST 3rd RevisionLavanya Bhat100% (1)
- Ps 1144BL-4WD 1Document53 pagesPs 1144BL-4WD 1Gheorghe HolteaNo ratings yet
- 666 GenderDocument4 pages666 Gendermozollis22No ratings yet
- Flow Computer and Loading Control SystemDocument14 pagesFlow Computer and Loading Control SystemAzito Jum100% (1)
- MyExperiencewithTRUELOVE PDFDocument111 pagesMyExperiencewithTRUELOVE PDFThiyagu ThiyaguNo ratings yet
- 4V Circuit DiagramDocument1 page4V Circuit DiagramwjcbaaNo ratings yet
- Astrology, Vedic, Eook) - Gayatri Devi Vasudev - Art of Matching Charts (OCR)Document178 pagesAstrology, Vedic, Eook) - Gayatri Devi Vasudev - Art of Matching Charts (OCR)virpara100% (12)
- 6-18-7 Flow Meters and Pressure InstrumentsDocument9 pages6-18-7 Flow Meters and Pressure InstrumentssudokuNo ratings yet