Professional Documents
Culture Documents
3.5 Spatting of A: Hand
3.5 Spatting of A: Hand
Uploaded by
Kamleshkekane1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
3.5 Spatting of A: Hand
3.5 Spatting of A: Hand
Uploaded by
Kamleshkekane1Copyright:
Available Formats
3.
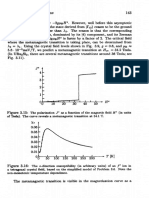
5 Spatting of a d-Level in Cubic Field 99
Fi e 3.5: Left and middle: High-spin and law-spin configurations of a CoS+ (or
P
Fe +) ion in cubic environment. Right: In LaMnO3, the Mn3+ ion preserves its high-
spin state but the large crystal field splitting leads to a clear disctinction between
“core” ( t s o ) and “lightly bound* (eg) electrons.
would predict the configuration t&ei, with S = 2; we may call this the
high-spin state (Fig. 3.5). On the other hand, with six &electrons one
can just fill the t2, level, thereby avoiding the occupation of eg states.
The configuration t!g goes with S = 0; this is the low-spin state. The
t2,-eg gap is the largest of crystal-field splittings, and it is apparently
large enough to compete with the intra-atomic exchange energy which
favours S = 2. In nature, sometimes the high-spin state, and some-
times the low-spin state is found to be stable. A careful investigation
of LiC002 provided a clear-cut case of the low-spin state of Co3+ [421].
We may ask whether the competition between high-spin and low-
spin states does not extend to the 3d7 configuration where S = 3/2
is the high spin and S = 1/2 would be the low spin. Indeed it has
been suggested that Ni3+ is in its S = 112 low-spin state in the curious
antifmomagnet LiNiO2.
The perovskite Lac003 is an interesting system where the low-spin
and high-spin configurations have comparable stability, and therefore
both are seen in the range of accessible temperatures. Given that the
low-spin configuration is slightly lower in energy (which seems to be the
cme), we. can think of at least two reasons why the high-spin configu-
ration should appear in large concentration at elevated temperatures.
One irr the large spin entropy associated with S = 2. Another is that as
soon aa the ions acquire spin, they can gain energy from spin-spin inter-
actions, or may even order magnetically. We may be wondering whether
You might also like
- Etkin Dynamics of Atmospheric Flight PDFDocument2 pagesEtkin Dynamics of Atmospheric Flight PDFJessica100% (1)
- SKF Hydraulic CouplingsDocument32 pagesSKF Hydraulic CouplingstanasaNo ratings yet
- Work, Energy and PowerDocument7 pagesWork, Energy and PowerBiprodeep14No ratings yet
- 3.6 Jahn-Teller Effect: 2 High-Spin State (FigDocument1 page3.6 Jahn-Teller Effect: 2 High-Spin State (FigKamleshkekane1No ratings yet
- Of Has: 'It Is Called Low-Spin in Comparison To 5/2 Which Is The Largest Possible Value The Spin ThisDocument1 pageOf Has: 'It Is Called Low-Spin in Comparison To 5/2 Which Is The Largest Possible Value The Spin ThisSandipNo ratings yet
- CFT PDFDocument20 pagesCFT PDFRUFAS KANIKANTINo ratings yet
- Review ArticleDocument13 pagesReview ArticleMuhammad IrfanNo ratings yet
- T. Masuda Et Al - Cooperative Ordering of Gapped and Gapless Spin Networks in Cu2Fe2Ge4O13Document4 pagesT. Masuda Et Al - Cooperative Ordering of Gapped and Gapless Spin Networks in Cu2Fe2Ge4O13Tellusz4532No ratings yet
- Ch. 5 Mott Insulators: "The Lal-,Sr, Tio3 System Will Discussed Detail See. 10.5Document1 pageCh. 5 Mott Insulators: "The Lal-,Sr, Tio3 System Will Discussed Detail See. 10.5KetanNo ratings yet
- In The: y T, WithDocument1 pageIn The: y T, WithSupriyaNo ratings yet
- J For A A: CodgumtionDocument1 pageJ For A A: CodgumtionKamleshkekane1No ratings yet
- 00131Document1 page00131Kamleshkekane1No ratings yet
- Is Idea An: OrbitalDocument1 pageIs Idea An: OrbitalKetanNo ratings yet
- Sol4 99Document9 pagesSol4 99HossainNo ratings yet
- CFTDocument15 pagesCFTGaurav BothraNo ratings yet
- 3 Field: FieldsDocument1 page3 Field: FieldsKamleshkekane1No ratings yet
- Jahn-Tkller Effect: It A at A atDocument1 pageJahn-Tkller Effect: It A at A atKamleshkekane1No ratings yet
- Nuclear IsomersDocument9 pagesNuclear Isomerskkl12No ratings yet
- Exchange Induced Magnetism: Irtl)Document1 pageExchange Induced Magnetism: Irtl)Kamleshkekane1No ratings yet
- And Kondo: Kfr/R2Document1 pageAnd Kondo: Kfr/R2SupriyaNo ratings yet
- 5.3 Orbital: 227 Is LowerDocument1 page5.3 Orbital: 227 Is LowerKetanNo ratings yet
- Ca, SR, A: Ferromagnetism inDocument1 pageCa, SR, A: Ferromagnetism inSupriyaNo ratings yet
- Atomic B1 ProblemsDocument8 pagesAtomic B1 ProblemsSimon Maxwell-StewartNo ratings yet
- Crystal Field: TheoryDocument1 pageCrystal Field: TheoryKamleshkekane1No ratings yet
- Kondo: A 0. A So ADocument1 pageKondo: A 0. A So ASupriyaNo ratings yet
- Electronic Band Structure and Lattice Distortion in Perovskite Transition-Metal OxidesDocument3 pagesElectronic Band Structure and Lattice Distortion in Perovskite Transition-Metal Oxidesanon_452078312No ratings yet
- Frustrated Magnetism in The Quantum Kagome Herbertsmithite Zncu3 (Oh) 6Cl2 AntiferromagnetDocument9 pagesFrustrated Magnetism in The Quantum Kagome Herbertsmithite Zncu3 (Oh) 6Cl2 AntiferromagnetHim HoangNo ratings yet
- Electronic Spectra of ComplexesDocument82 pagesElectronic Spectra of Complexesirembasar2000No ratings yet
- IJ S (S: Ferromagnetic Heisenberg ModelDocument1 pageIJ S (S: Ferromagnetic Heisenberg ModelKetanNo ratings yet
- Chalker FinalDocument44 pagesChalker FinalMuhammad Shifan JayadiNo ratings yet
- Magnetic Phase Transition and Spin Wave ExcitationDocument12 pagesMagnetic Phase Transition and Spin Wave ExcitationKiki Rezki LestariNo ratings yet
- T. C. Killian Et Al - Formation of Rydberg Atoms in An Expanding Ultracold Neutral PlasmaDocument4 pagesT. C. Killian Et Al - Formation of Rydberg Atoms in An Expanding Ultracold Neutral PlasmaItama23No ratings yet
- Crystal Field Theory (CFT)Document15 pagesCrystal Field Theory (CFT)veronicaNo ratings yet
- AS K AS: MagnetsDocument1 pageAS K AS: MagnetsKetanNo ratings yet
- 2011 MPSD Qcm+et - Petersen PRLDocument5 pages2011 MPSD Qcm+et - Petersen PRLnickydean83No ratings yet
- Insulators: Ch. 5 MottDocument1 pageInsulators: Ch. 5 MottKetanNo ratings yet
- Exam 1 RepeatDocument7 pagesExam 1 RepeatBakhita MaryamNo ratings yet
- J Hep 021999011Document24 pagesJ Hep 021999011ksva2326No ratings yet
- 1.3 Getting Acquainted: Magnetite: 1.3.2 SpinDocument1 page1.3 Getting Acquainted: Magnetite: 1.3.2 SpinSandipNo ratings yet
- Coord. Chem Notes - Modified 09-02-2023-1Document11 pagesCoord. Chem Notes - Modified 09-02-2023-1Ebsiba Beaula JNo ratings yet
- PhysRevLett 70 414Document4 pagesPhysRevLett 70 414saves-03balladsNo ratings yet
- Gas Dynamics in Clusters of GalaxiesDocument30 pagesGas Dynamics in Clusters of GalaxiespjblkNo ratings yet
- Superconductivity, A Physical Chemical Perspective: Ralph C. DoughertyDocument36 pagesSuperconductivity, A Physical Chemical Perspective: Ralph C. Doughertyjuan lopezNo ratings yet
- Chapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismDocument13 pagesChapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismAlia AliaNo ratings yet
- Induced and Remanent MagnetismDocument14 pagesInduced and Remanent MagnetismArnaldo Hernández CardonaNo ratings yet
- Field: Lgremember That in This Discussion, Is Measured in Units of Thus The Ordered MomentDocument1 pageField: Lgremember That in This Discussion, Is Measured in Units of Thus The Ordered MomentKetanNo ratings yet
- How High The Spin Allowed and Forbidden Spin StatesDocument9 pagesHow High The Spin Allowed and Forbidden Spin StatesThiago Costa SerraNo ratings yet
- Younker 2013Document10 pagesYounker 2013Anonymous ZY43E2DTNo ratings yet
- A A I and 0 As Its: 3 Crystal Field The01 A. ThisDocument1 pageA A I and 0 As Its: 3 Crystal Field The01 A. ThisKamleshkekane1No ratings yet
- So Where Do We Begin? Atomic & Ionic Radii - 1Document6 pagesSo Where Do We Begin? Atomic & Ionic Radii - 1NziluNo ratings yet
- Lab 4 - MODULE γ-2: 3.014 Materials Laboratory Dec. 8 - Dec..13, 2006Document8 pagesLab 4 - MODULE γ-2: 3.014 Materials Laboratory Dec. 8 - Dec..13, 2006ALEXANDER DAVID PARICELA CRUZNo ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- PhysRevD 101 103535aquimodelDocument11 pagesPhysRevD 101 103535aquimodelMartin WolffeNo ratings yet
- Features of Excess Conductivity Behavior in A Magnetic Superconductor Dy0.6Y0.4Rh3.85Ru0.15B4Document10 pagesFeatures of Excess Conductivity Behavior in A Magnetic Superconductor Dy0.6Y0.4Rh3.85Ru0.15B4cibin35477No ratings yet
- 1 IntroductoryDocument45 pages1 IntroductoryTuhin Sahu100% (1)
- Chapter-2B: Dr. The ThermalDocument9 pagesChapter-2B: Dr. The Thermalkoulickchakraborty5555No ratings yet
- Woestenenk NimbDocument10 pagesWoestenenk NimbFundaljohnNo ratings yet
- R.F Of: Theory of Color Centers in Ionic Crystals. IIDocument10 pagesR.F Of: Theory of Color Centers in Ionic Crystals. IIDikshit GautamNo ratings yet
- The Solid State: Band Theory of SolidsDocument31 pagesThe Solid State: Band Theory of SolidsVinsen Teubun LetsoinNo ratings yet
- EsrDocument5 pagesEsrVirendra Singh Rajput100% (1)
- A Have: Anisotropy Splittizzgs TheDocument1 pageA Have: Anisotropy Splittizzgs TheKamleshkekane1No ratings yet
- The Fact: A of Is LowerDocument1 pageThe Fact: A of Is LowerSupriyaNo ratings yet
- Amorphous Semiconductors: Structural, Optical, and Electronic PropertiesFrom EverandAmorphous Semiconductors: Structural, Optical, and Electronic PropertiesNo ratings yet
- Hubbard: and ModelDocument1 pageHubbard: and ModelKamleshkekane1No ratings yet
- It At: Hubbard ModelDocument1 pageIt At: Hubbard ModelKamleshkekane1No ratings yet
- Hubbard Model: ModelsDocument1 pageHubbard Model: ModelsKamleshkekane1No ratings yet
- Mott Llansition: Is IsDocument1 pageMott Llansition: Is IsKamleshkekane1No ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- # (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelDocument1 page# (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Transition: Is COO A ofDocument1 pageTransition: Is COO A ofKamleshkekane1No ratings yet
- 4.2 Mott Damition: Na 4a. CriticalDocument1 page4.2 Mott Damition: Na 4a. CriticalKamleshkekane1No ratings yet
- It Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityDocument1 pageIt Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityKamleshkekane1No ratings yet
- A3s At: Mott Transition and Hubbard ModelDocument1 pageA3s At: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- Mott Transition and Hubbard Model: Right: SchematicDocument1 pageMott Transition and Hubbard Model: Right: SchematicKamleshkekane1No ratings yet
- And Hubbard Model: To (FS)Document1 pageAnd Hubbard Model: To (FS)Kamleshkekane1No ratings yet
- Mott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionDocument1 pageMott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionKamleshkekane1No ratings yet
- A $ (BLT) A: Crystal Field TheoryDocument1 pageA $ (BLT) A: Crystal Field TheoryKamleshkekane1No ratings yet
- 144 3 Crystal: H Ma3h"Document1 page144 3 Crystal: H Ma3h"Kamleshkekane1No ratings yet
- Field: Ch. 3 Crystal TheoryDocument1 pageField: Ch. 3 Crystal TheoryKamleshkekane1No ratings yet
- Solutions The: So Are ToDocument1 pageSolutions The: So Are ToKamleshkekane1No ratings yet
- Solutions To The Problems: FreeDocument1 pageSolutions To The Problems: FreeKamleshkekane1No ratings yet
- Crystal: Field TheoryDocument1 pageCrystal: Field TheoryKamleshkekane1No ratings yet
- Jar, JZ,: Solutiom To The ProblemsDocument1 pageJar, JZ,: Solutiom To The ProblemsKamleshkekane1No ratings yet
- Solutions To The Problems 143: H,, 34.2 TeslaDocument1 pageSolutions To The Problems 143: H,, 34.2 TeslaKamleshkekane1No ratings yet
- Crystal Field Theory: in IsDocument1 pageCrystal Field Theory: in IsKamleshkekane1No ratings yet
- @Q/&GFQGZ.: Solutions To The ProblemsDocument1 page@Q/&GFQGZ.: Solutions To The ProblemsKamleshkekane1No ratings yet
- t2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryDocument1 paget2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryKamleshkekane1No ratings yet
- Phsics Solutions CH 4 2Document26 pagesPhsics Solutions CH 4 2Stephanie TeoNo ratings yet
- Department of Chemical Engineering School of Engineering and Architecture Saint Louis University Laboratory Report Evaluation SheetDocument18 pagesDepartment of Chemical Engineering School of Engineering and Architecture Saint Louis University Laboratory Report Evaluation SheetSarah SanchezNo ratings yet
- Physics Tut Sheet 3 Dielectric PropertiesDocument2 pagesPhysics Tut Sheet 3 Dielectric PropertiesHarshit SoniNo ratings yet
- Use of Lba-Mna Methodology For Determination of Bearing Capacity of Compressed Shells Lyubomir ZdravkovDocument6 pagesUse of Lba-Mna Methodology For Determination of Bearing Capacity of Compressed Shells Lyubomir ZdravkovnithinjcNo ratings yet
- Affleck Dine MechanismDocument38 pagesAffleck Dine MechanismAshla MuadNo ratings yet
- GMSH TutorialDocument8 pagesGMSH TutorialGeorge CarmelNo ratings yet
- Marc TrussDocument19 pagesMarc Trussganesh_withucadNo ratings yet
- Volume Effect in The Landau Theory of Martensitic Phase Transitions in Cubic CrystalsDocument39 pagesVolume Effect in The Landau Theory of Martensitic Phase Transitions in Cubic CrystalsPrevalisNo ratings yet
- Tribology Aspects in Angular Transmission Systems: Hypoid GearsDocument7 pagesTribology Aspects in Angular Transmission Systems: Hypoid GearspiruumainNo ratings yet
- Chapter - 3 Rapidly Varied FlowDocument8 pagesChapter - 3 Rapidly Varied FlowAbdulrasheed BashirNo ratings yet
- Example Pipe Flow Problems SOLUTIONSDocument10 pagesExample Pipe Flow Problems SOLUTIONSbest contentNo ratings yet
- 8fc63d2f f6Document18 pages8fc63d2f f6Chandra MohanNo ratings yet
- 1 Baker Cheli Cross Wind Effects On Read and Rail VehiclesDocument41 pages1 Baker Cheli Cross Wind Effects On Read and Rail VehiclesDoicielNo ratings yet
- PLAXIS - 2D - CEV21 - Tutorial - 10 - Cyclic Vertical Capacity and Stiffness of Circular Underwater FootingDocument21 pagesPLAXIS - 2D - CEV21 - Tutorial - 10 - Cyclic Vertical Capacity and Stiffness of Circular Underwater FootingjayawiadnyanaNo ratings yet
- TribologyDocument8 pagesTribologyKamal ChaitanyaNo ratings yet
- Distance and Displacement QuizDocument4 pagesDistance and Displacement QuizSarah Joy TadejaNo ratings yet
- Sigma 1 14-User ManualDocument64 pagesSigma 1 14-User ManualLucila Figueroa GalloNo ratings yet
- 370 The Red Book - Basics of Foundation Design Fellenius 2017 PDFDocument468 pages370 The Red Book - Basics of Foundation Design Fellenius 2017 PDFTomasz Cz100% (1)
- Synchronous Machine Pu ModelDocument11 pagesSynchronous Machine Pu ModelDinesh ShettyNo ratings yet
- Thermodynamic Consideration Production of Ethyl BenzeneDocument3 pagesThermodynamic Consideration Production of Ethyl BenzeneCer No RusNo ratings yet
- CHEM113 Assignment No1Document3 pagesCHEM113 Assignment No1Mark Ryan TripoleNo ratings yet
- Dislocations and Plastic DeformationDocument6 pagesDislocations and Plastic DeformationPrashanth VantimittaNo ratings yet
- Irp DPP No7Document4 pagesIrp DPP No7Shivam JaggiNo ratings yet
- J Halling Principles of TribologyDocument414 pagesJ Halling Principles of Tribologylokesh narendhiranNo ratings yet
- Calculator For Finding Principal Strains PDFDocument3 pagesCalculator For Finding Principal Strains PDFWEVVNo ratings yet
- Integral Relations For A Control Volume: Boundary SurroundingsDocument7 pagesIntegral Relations For A Control Volume: Boundary Surroundingsاحمد عبد المحسن ناجيNo ratings yet
- Physics: Unit: 4PH0 Science (Double Award) 4SC0 Paper: 1PRDocument32 pagesPhysics: Unit: 4PH0 Science (Double Award) 4SC0 Paper: 1PRMissKa's GardenNo ratings yet