Professional Documents
Culture Documents
A3s At: Mott Transition and Hubbard Model
A3s At: Mott Transition and Hubbard Model
Uploaded by
Kamleshkekane1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A3s At: Mott Transition and Hubbard Model
A3s At: Mott Transition and Hubbard Model
Uploaded by
Kamleshkekane1Copyright:
Available Formats
152 Ch.
4 Mott Transition and Hubbard Model
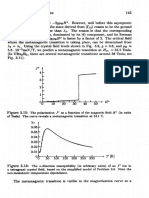
In the first line, the process of electron hopping is schematically shown.
In the second line, we give the corresponding energies of the neutral,
and ionized configurations, respectively. An electron sitting in a 3s state
possesses the atomic energy ~ 3 If~two . electrons share the same atomic
shell, as at the Na- site shown on the right-hand side, their strong
Coulomb repulsion goes with the intraatomic Coulomb energy7
This allows us to deduce that electron hopping requires the energy in-
vestment U3s.Since sharing the same atomic orbital confines the two
electrons to within a short distance ( w 181) from each other, must
be large.
The intraatomic Coulomb energy, usually called the Hubbard U ,is
the most important energy term for a system of interacting electrons
moving in a narrow band. For various atomic orbitals in a solid state
environment, its typical range is 1-lOeV. The charge transfer process
shown in (4.2)costs' a lot of energy so it just does not want to happen.
The charge fluctuations necessary for metallic conduction are suppressed
by the intrasite Coulomb interaction - at least above some critical valueg
of the lattice constant (i.e., for sufficiently narrow bands). This transi-
tion from a metallic to an insulating state would be an example of the
Mott transition.
The energy terms needed to discuss the Gedankenexperiment in
Fig. 4.1 can be collected in the following simple Hamiltonian
j ' till)
= %band -t%Coulomb (4.4)
'The analogous quantity for the hydrogen molecule wa8 introduced in (2.128).
'For Na, this would be larger than the equilibrium lattice constant (so the system
remains, in fact, a metal) but not absurdly larger. We have in mind interatomic
distances several As. - Essentially the same argument was put forward in 1937 by
N
R. Peierls to explain that NiO is an insulator. He argued that it is due to the large
Coulomb repulsion between the d-electrons, and that each Ni2+ ion keeps exactly 8
d-electrons, and only the spin degree of freedom survives. We have chosen to speak
about the hypothetical dilated Na because this leads on straight to the introduction
of the non-degenerate Hubbard model.
You might also like
- Semiconductor Theory and DevicesDocument105 pagesSemiconductor Theory and DevicesAnonymous AyCl4LNo ratings yet
- Open Structure of Atom Telegram PDFDocument8 pagesOpen Structure of Atom Telegram PDFnimarsharma7No ratings yet
- Chapter 1Document25 pagesChapter 1Mostar NNo ratings yet
- Current Ohmos: Electric LAWDocument25 pagesCurrent Ohmos: Electric LAWMostar NNo ratings yet
- Photovolatics Developments: A Review On Intermediate Band Solar Cells-Underlying Concepts and Latest DevelopmentsDocument18 pagesPhotovolatics Developments: A Review On Intermediate Band Solar Cells-Underlying Concepts and Latest DevelopmentsaqstaxNo ratings yet
- Ch02 AtomicNucleusDocument8 pagesCh02 AtomicNucleusPrichindel MorocanosNo ratings yet
- R.F Of: Theory of Color Centers in Ionic Crystals. IIDocument10 pagesR.F Of: Theory of Color Centers in Ionic Crystals. IIDikshit GautamNo ratings yet
- Semi ConductorDocument50 pagesSemi ConductorVibhor KaushikNo ratings yet
- Pauli TrapDocument6 pagesPauli TrapMichael MurphyNo ratings yet
- HallDocument29 pagesHallValen SanchezNo ratings yet
- The Royal SocietyDocument20 pagesThe Royal SocietyJardel da RosaNo ratings yet
- Referee ReportDocument6 pagesReferee ReportIngenioerenNo ratings yet
- Bohr and Rydberg - Atomic Spectra ProblemsDocument13 pagesBohr and Rydberg - Atomic Spectra ProblemssubstitutescribdNo ratings yet
- Lecture3 Module 5Document7 pagesLecture3 Module 5chinmoyd988No ratings yet
- Free Electron TheryDocument7 pagesFree Electron TheryRNo ratings yet
- Hubbard Model Forh2Document6 pagesHubbard Model Forh2Jardel da RosaNo ratings yet
- Material 2Document22 pagesMaterial 2zeekumNo ratings yet
- Module 113-Quantum Theory and Atomic Spectroscopy LECTURE 2 - Discrete Energy LevelsDocument9 pagesModule 113-Quantum Theory and Atomic Spectroscopy LECTURE 2 - Discrete Energy Levelsapi-19928045No ratings yet
- Lab Report-2 - 18-36652-1Document11 pagesLab Report-2 - 18-36652-1Zahid Hasan KhokaNo ratings yet
- Physis 2nd and 3rd Term ss3Document38 pagesPhysis 2nd and 3rd Term ss3Dada RasheedNo ratings yet
- Material Chapter TwoDocument9 pagesMaterial Chapter TwoTeshale AlemieNo ratings yet
- Solid Short Notes PDFDocument32 pagesSolid Short Notes PDFsanjeet singh kainturaNo ratings yet
- Chapter 3.4, Many-Electron Atoms: Fermi Holes and Fermi HeapsDocument14 pagesChapter 3.4, Many-Electron Atoms: Fermi Holes and Fermi HeapsBoceNo ratings yet
- CBSE Class 12 Physics: Atoms and Nuclei QuestionsDocument4 pagesCBSE Class 12 Physics: Atoms and Nuclei QuestionsBug LordNo ratings yet
- Bohr's Model, Energy Bands, Electrons and Holes: Dual Character of Material ParticlesDocument30 pagesBohr's Model, Energy Bands, Electrons and Holes: Dual Character of Material ParticlespurseyNo ratings yet
- P 62Document25 pagesP 62JohnnardBelenNo ratings yet
- Conductor Materials: 4.1 Definitions and General PropertiesDocument9 pagesConductor Materials: 4.1 Definitions and General PropertiesAdriana PetrieNo ratings yet
- Chapter IiiDocument15 pagesChapter Iiidedy krisnayanaNo ratings yet
- Microwave Semiconductor Device Technologies 4. Energy Bands and Charge CarrierDocument11 pagesMicrowave Semiconductor Device Technologies 4. Energy Bands and Charge Carriersushil4056No ratings yet
- Semiconductors: ELEC 353 1Document25 pagesSemiconductors: ELEC 353 1Ankur MaheshwariNo ratings yet
- Drude Model PDFDocument5 pagesDrude Model PDFkeshav sharmaNo ratings yet
- Part 1. Background Material: Chapter 1. The Basics of Quantum MechanicsDocument94 pagesPart 1. Background Material: Chapter 1. The Basics of Quantum MechanicsKirby BurneaNo ratings yet
- Ch-2 Chemistry (Structure of Atom) Class-11Document10 pagesCh-2 Chemistry (Structure of Atom) Class-11kartikaryan9250No ratings yet
- Bohrs Theory of The Hydrogen Atom 6Document16 pagesBohrs Theory of The Hydrogen Atom 6FerdiAhmadNo ratings yet
- Band Theory of SolidsDocument12 pagesBand Theory of SolidsFitrianiNo ratings yet
- Atomic StructureDocument10 pagesAtomic StructureSatyam MittalNo ratings yet
- Final HallDocument29 pagesFinal HallUnknownNo ratings yet
- Semiconductor Physics 3Document60 pagesSemiconductor Physics 3abinaya050794No ratings yet
- Reduced Mass Effect in Hydrogen Atom PDFDocument6 pagesReduced Mass Effect in Hydrogen Atom PDFNiraj KumarNo ratings yet
- Unit IVDocument18 pagesUnit IVSruthi ShineyNo ratings yet
- Blake - 1981 - Exchange Stabilization and The Variation of Ionization Energy in The PN and DN SeriesDocument6 pagesBlake - 1981 - Exchange Stabilization and The Variation of Ionization Energy in The PN and DN SeriesNikole EspinozaNo ratings yet
- Physics 244 Notes Doped SemiconductorsDocument4 pagesPhysics 244 Notes Doped SemiconductorsChichia DollNo ratings yet
- Electron Correlations in Narrow Energy BandsDocument20 pagesElectron Correlations in Narrow Energy BandsJoy RoyNo ratings yet
- Chapter One Atomic SturctureDocument7 pagesChapter One Atomic SturctureWorld ShortsNo ratings yet
- Lokesh ProjectDocument14 pagesLokesh ProjectToxicNo ratings yet
- 271 - PH8252 Physics For Information Science - Notes UNIT I ELECTRICAL PROPERTIES OF MATERIALSDocument39 pages271 - PH8252 Physics For Information Science - Notes UNIT I ELECTRICAL PROPERTIES OF MATERIALSisaacasimov1304No ratings yet
- 12 AtomsDocument18 pages12 Atomsbrainbots0No ratings yet
- S.A. Kivelson Et Al - 2e or Not 2e: Flux Quantization in The Resonating Valence Bond StateDocument6 pagesS.A. Kivelson Et Al - 2e or Not 2e: Flux Quantization in The Resonating Valence Bond StatePo48HSDNo ratings yet
- Bohr 1913 PDFDocument26 pagesBohr 1913 PDFOrhun Özer100% (1)
- Physics Unit 1Document131 pagesPhysics Unit 17csnty5wvgNo ratings yet
- Unit 1 Semiconductor Lecture NotesDocument22 pagesUnit 1 Semiconductor Lecture NotesMy Extra AccountNo ratings yet
- J.N. Murrell, J. Tennyson and M.A. Kamel - Many-Body Contributions To The Intermolecular Potential in Alkali Halide Crystals and ClustersDocument9 pagesJ.N. Murrell, J. Tennyson and M.A. Kamel - Many-Body Contributions To The Intermolecular Potential in Alkali Halide Crystals and ClustersMaxnamewNo ratings yet
- Ak Electronic Devices Unit 1Document8 pagesAk Electronic Devices Unit 1Shirin RazdanNo ratings yet
- Unit I Electrical Properties of Materials PDFDocument39 pagesUnit I Electrical Properties of Materials PDFSankar RamNo ratings yet
- Resistivity Four ProbeDocument23 pagesResistivity Four ProbeK.H. TanNo ratings yet
- 4.unit IV Basic Quantum PhysicsDocument24 pages4.unit IV Basic Quantum Physicsjoshua18012005No ratings yet
- HybridizationDocument9 pagesHybridizationSatyaki MajumdarNo ratings yet
- Atomic Structure JEE Main and Advanced (Theory)Document19 pagesAtomic Structure JEE Main and Advanced (Theory)Er. Vineet Loomba (IIT Roorkee)No ratings yet
- Electronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestFrom EverandElectronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestNo ratings yet
- Of of For: FieldDocument1 pageOf of For: FieldKamleshkekane1No ratings yet
- It At: Hubbard ModelDocument1 pageIt At: Hubbard ModelKamleshkekane1No ratings yet
- Hubbard Model: ModelsDocument1 pageHubbard Model: ModelsKamleshkekane1No ratings yet
- # (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelDocument1 page# (R RJ) With Spin J Being A Lattice Site Index. Cjucju: Mott Transition and Hubbard ModelKamleshkekane1No ratings yet
- 4.2 Mott Damition: Na 4a. CriticalDocument1 page4.2 Mott Damition: Na 4a. CriticalKamleshkekane1No ratings yet
- And Hubbard Model: To (FS)Document1 pageAnd Hubbard Model: To (FS)Kamleshkekane1No ratings yet
- Transition: Is COO A ofDocument1 pageTransition: Is COO A ofKamleshkekane1No ratings yet
- Mott Llansition: Is IsDocument1 pageMott Llansition: Is IsKamleshkekane1No ratings yet
- Hubbard: and ModelDocument1 pageHubbard: and ModelKamleshkekane1No ratings yet
- Mott Transition and Hubbard Model: Right: SchematicDocument1 pageMott Transition and Hubbard Model: Right: SchematicKamleshkekane1No ratings yet
- 144 3 Crystal: H Ma3h"Document1 page144 3 Crystal: H Ma3h"Kamleshkekane1No ratings yet
- It Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityDocument1 pageIt Na Atoms, A A: Is Clear That Eventually This Leads To An AbsurdityKamleshkekane1No ratings yet
- Jar, JZ,: Solutiom To The ProblemsDocument1 pageJar, JZ,: Solutiom To The ProblemsKamleshkekane1No ratings yet
- Mott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionDocument1 pageMott Transition Hubbard Model: Metals and Insulators: Breakdown The Independent-Electron DescriptionKamleshkekane1No ratings yet
- A $ (BLT) A: Crystal Field TheoryDocument1 pageA $ (BLT) A: Crystal Field TheoryKamleshkekane1No ratings yet
- Crystal Field Theory: in IsDocument1 pageCrystal Field Theory: in IsKamleshkekane1No ratings yet
- Solutions To The Problems: FreeDocument1 pageSolutions To The Problems: FreeKamleshkekane1No ratings yet
- Crystal: Field TheoryDocument1 pageCrystal: Field TheoryKamleshkekane1No ratings yet
- @Q/&GFQGZ.: Solutions To The ProblemsDocument1 page@Q/&GFQGZ.: Solutions To The ProblemsKamleshkekane1No ratings yet
- Solutions To The Problems 143: H,, 34.2 TeslaDocument1 pageSolutions To The Problems 143: H,, 34.2 TeslaKamleshkekane1No ratings yet
- Field: Ch. 3 Crystal TheoryDocument1 pageField: Ch. 3 Crystal TheoryKamleshkekane1No ratings yet
- Solutions The: So Are ToDocument1 pageSolutions The: So Are ToKamleshkekane1No ratings yet
- t2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryDocument1 paget2, Y ' - (Y," - Y ) - y . E, Yz" &Y YF2) .: Ch. 3 Crystal Field TheoryKamleshkekane1No ratings yet