Professional Documents
Culture Documents
TechChem Lab 2
TechChem Lab 2
Uploaded by
Marzouki EyaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
TechChem Lab 2
TechChem Lab 2
Uploaded by
Marzouki EyaCopyright:
Available Formats
Khlopetska Viktoriia, P893B0, Group B
Laboratory Report
Electrochemical Corrosion of Metals
1. Determination of corrosion potentials
Measure the electrode potentials of various metals and metal alloys compared to calomel
electrode in the 2% NaCl solution.
- Place the metal to be measured in the 2% NaCl solution and the calomel reference electrode.
- Measure the electrode potential between them by a multimeter one minute after the loop
linking.
- Read the measured value with correct sign and note with proper unit (0.01 Volt).
- Remove the metal from the electrolyte, wash and wipe it dry.
- Repeat the measurement with a new metal.
Metals included: Zn bar, Zn, Cu bar, Cu, Fe, Pb, Graphite, Al
Test results
Metal Electric potential (V)
Zn bar -0.91
Zn -0.95
Cu bar -0.12
Cu -0.15
Fe -0.42
Pb -0.495
Graphite +0.117
Al -0.726
2. Determination of corrosion rate
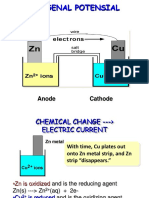
- Select two metal – Zn and Al, immerse both into the 2% NaCl solution.
- Measure the electric current accompanying the corrosion process between the two metals by
the multimeter properly connected. After approximately two minutes, record the measured
current in proper unit.
- Identify using the potentials obtained during the previous measurement data, that which
metal will be expected to suffer corrosion – in our case Zn.
- Measure the current
I = 0,7 mA = 0,0007 A
- Measure the metal surface, which is expected to suffer from corrosion.
A = 2*2*4,5 = 14 cm2 = 0,0014 m2
- Calculate the corrosion rate [g/m2year] and [mm/year] in units.
The amount of metal went into solution can be determined by the Faraday law:
atomic mass of dissolving metal
m = k * I * t where k = --------------------------------
(z*F)
where m : mass of metal went into solution [g]
k : metal dependent Faraday constans [g/Ah]
I : corrosion current [A]
z : charge of the metallic ion went into solution
F : Faraday constant 26,8 [Ah]
t : duration of the corrosion process [hour]
k = M / z*F = 65,37 / 2*26,8 = 1,2196
m = 1,2196*0,0007*1 = 0.00085 g/hour (*8766) = 7,48 g/year
Vcorr = 7,48/0,0014 = 5342 g/(m2year)
Vlinear corr = 5342/7140000 = 0,00075 m/year = 7,5 mm/year
You might also like
- Sample Storyboard For CoursewareDocument8 pagesSample Storyboard For CoursewareMichael SkyersNo ratings yet
- Electro Chemistry FinalDocument51 pagesElectro Chemistry FinalManoj50% (2)
- Exp 2 Electrochemistry - Electrochemical Cell and Thermodynamic FunctionsDocument8 pagesExp 2 Electrochemistry - Electrochemical Cell and Thermodynamic FunctionsMuhammad Amirul AfifiNo ratings yet
- Act03 Exploring ElectrochemistryDocument13 pagesAct03 Exploring ElectrochemistryRenNo ratings yet
- Asl WorkbookDocument11 pagesAsl WorkbookSonal SinglaNo ratings yet
- Electrochemistry Lab Apr2023Document6 pagesElectrochemistry Lab Apr2023Enock KamugishaNo ratings yet
- ElectrochemistryDocument74 pagesElectrochemistryVipranshu GuptaNo ratings yet
- Practical 1 ElectrolysisDocument27 pagesPractical 1 ElectrolysisGeorge chaupi NyondoNo ratings yet
- Construction of Ag - AgCl Reference Electrode and ApplicationDocument3 pagesConstruction of Ag - AgCl Reference Electrode and ApplicationValentin-AngeloUzunovNo ratings yet
- Chm221 Chapter 5Document42 pagesChm221 Chapter 5Badrudin JundailiNo ratings yet
- Electrochem: Battery Project: By: Dimitrije Randjelovic and Ryan FoskeyDocument9 pagesElectrochem: Battery Project: By: Dimitrije Randjelovic and Ryan FoskeyDimitrije RandjelovicNo ratings yet
- CH 142 Exp 8 ElectroplatingDocument10 pagesCH 142 Exp 8 ElectroplatingArely CárdenasNo ratings yet
- Electrogravimetry and CoulometryDocument53 pagesElectrogravimetry and CoulometrySunil Kumar100% (3)
- Electrochemistry 1Document74 pagesElectrochemistry 1Vipranshu GuptaNo ratings yet
- Experiment 1A:: Determining The Voltage of The Voltaic CellDocument5 pagesExperiment 1A:: Determining The Voltage of The Voltaic Cellhusna azizanNo ratings yet
- Electrochemical CellDocument5 pagesElectrochemical Cellrekhadohrey450No ratings yet
- Lab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellDocument11 pagesLab Report: Cmt555: Experiment 1: Galvanic & Electrolytic CellkuekNo ratings yet
- Revision - Metals and Electrolysis - AdjustedDocument10 pagesRevision - Metals and Electrolysis - AdjustedLiew You tong (Unityss)No ratings yet
- MODULE 3-Dr - HariDocument111 pagesMODULE 3-Dr - HariKartik KaushikNo ratings yet
- Corrosion Monitoring PrimerDocument61 pagesCorrosion Monitoring PrimerShubhodeep SarkarNo ratings yet
- Module 1Document15 pagesModule 1venugopal_aeroNo ratings yet
- RJ Corrosion FullDocument73 pagesRJ Corrosion FullSai MedaNo ratings yet
- Lab Report Corrosion-1Document10 pagesLab Report Corrosion-1areniqwardiah_918730100% (1)
- Discovering Electrochemical Cells: PGCC CHM 102 SinexDocument36 pagesDiscovering Electrochemical Cells: PGCC CHM 102 Sinexavi0341No ratings yet
- Bab 19 Elektrokimia 1Document59 pagesBab 19 Elektrokimia 1Mohammad HamamNo ratings yet
- Unit-3 Chemical MethodsDocument99 pagesUnit-3 Chemical MethodsAkshay NachappaNo ratings yet
- Faraday'S LAW AND GALVANIC CELL LESSONDocument4 pagesFaraday'S LAW AND GALVANIC CELL LESSONmmmmmNo ratings yet
- Corrosion Lecture ManchesterDocument88 pagesCorrosion Lecture ManchesterAli AbbasovNo ratings yet
- Electrochemical ThermodynamicsDocument38 pagesElectrochemical ThermodynamicsikamelyaastutiNo ratings yet
- (실험7) Electrochemical cell and electrochemical seriesDocument14 pages(실험7) Electrochemical cell and electrochemical seriesinpiniti1234No ratings yet
- Powerpoint ElectrodepositionDocument24 pagesPowerpoint ElectrodepositionsobheysaidNo ratings yet
- Module 1 - Electrode Potential & CellsDocument13 pagesModule 1 - Electrode Potential & CellsrashmiNo ratings yet
- Experiment #2 Post Lab (Edited)Document8 pagesExperiment #2 Post Lab (Edited)Erwin CabangalNo ratings yet
- Environment Degradation of Materials - 1Document27 pagesEnvironment Degradation of Materials - 1NSHIMIYIMANA Jean d'AmourNo ratings yet
- Activity Wenzel Text Voltammetric MethodsDocument15 pagesActivity Wenzel Text Voltammetric MethodsLucica BarbesNo ratings yet
- Lab 8 Voltaic Cells Electrolytic Cells KM 2010Document9 pagesLab 8 Voltaic Cells Electrolytic Cells KM 2010Syazwan SallehNo ratings yet
- Electro Chemistry (MS)Document208 pagesElectro Chemistry (MS)Kaustubh SreekharNo ratings yet
- Introduction To Cathodic ProtectionDocument5 pagesIntroduction To Cathodic Protectionali AbbasNo ratings yet
- Electrochemistry 2019 HANDOUTDocument50 pagesElectrochemistry 2019 HANDOUTAndrearose Ivy FietasNo ratings yet
- Electrochemistry (CURRENT) - STDT1Document2 pagesElectrochemistry (CURRENT) - STDT1Nkemzi Elias NzetengenleNo ratings yet
- Electrochem 201516Document81 pagesElectrochem 201516Mohd AminudinNo ratings yet
- Unit 3 ElectrochemistryDocument47 pagesUnit 3 Electrochemistryabisheik942No ratings yet
- POTENSIAL KorosiDocument33 pagesPOTENSIAL Korosilisa andrianiNo ratings yet
- POTENSIAL KorosiDocument33 pagesPOTENSIAL KorosiLisa AndrianiNo ratings yet
- Electro Chemistry 2016 NEWDocument9 pagesElectro Chemistry 2016 NEWGaurav SharmaNo ratings yet
- On ElectrochemistryDocument27 pagesOn Electrochemistryfjym2vr7kbNo ratings yet
- 20BEC0739 Chem Construction of E CellDocument6 pages20BEC0739 Chem Construction of E CellSrenuBhonsleNo ratings yet
- Objectives: - Describe - Identify - Describe - CalculateDocument29 pagesObjectives: - Describe - Identify - Describe - CalculateJanaNo ratings yet
- Activity 7 ElectrochemistryDocument8 pagesActivity 7 ElectrochemistryEarl CagaananNo ratings yet
- Cathodic Protection LecDocument11 pagesCathodic Protection Lecali AbbasNo ratings yet
- Net Electrochemical CellsDocument21 pagesNet Electrochemical CellsSourav DasNo ratings yet
- ElectrolysisDocument21 pagesElectrolysisImranRazaBozdarNo ratings yet
- CHM 114 Exp 10Document6 pagesCHM 114 Exp 10João Antonio BassettoNo ratings yet
- Module 3Document107 pagesModule 3Anshu MalikNo ratings yet
- Lab 9 Electrochemical Cells and Cells PotentialsDocument9 pagesLab 9 Electrochemical Cells and Cells PotentialsaddislibroNo ratings yet
- 3.1 Permanent-Magnet Materials and CharacteristicsDocument34 pages3.1 Permanent-Magnet Materials and CharacteristicsHaripriya PalemNo ratings yet
- Lab Activity 7 ElectrochemistryDocument8 pagesLab Activity 7 Electrochemistryjhunjhun zambranoNo ratings yet
- ELECTROCHEMISTRYDocument176 pagesELECTROCHEMISTRYgsvssumaNo ratings yet
- Electrochem PPT 06.09.2023Document52 pagesElectrochem PPT 06.09.2023Jjo JioNo ratings yet
- Electrochemistry Part 3Document13 pagesElectrochemistry Part 3Shofwa AnnisaaNo ratings yet
- 2&3&4&5 Burner Induction+Infrared Cooker Quotations-Alan Liu (LONGSTAR)Document9 pages2&3&4&5 Burner Induction+Infrared Cooker Quotations-Alan Liu (LONGSTAR)Dương Nguyễn ĐắcNo ratings yet
- Your Results For: "Multiple Choice Questions": Always Receive Straight CommissionDocument4 pagesYour Results For: "Multiple Choice Questions": Always Receive Straight CommissionRaman KulkarniNo ratings yet
- An Evaluation of Wheel Chair Cum Bed Mechanism With Side Panel Movement For BedDocument6 pagesAn Evaluation of Wheel Chair Cum Bed Mechanism With Side Panel Movement For BedMuhammad Hammad Sabir PansotaNo ratings yet
- Coral ReefsDocument6 pagesCoral ReefsGia AN LêNo ratings yet
- PH of Soils: Standard Test Method ForDocument3 pagesPH of Soils: Standard Test Method ForYizel CastañedaNo ratings yet
- As 2610.1-2007 Spa Pools Public SpasDocument7 pagesAs 2610.1-2007 Spa Pools Public SpasSAI Global - APACNo ratings yet
- A Color Atlas of Poultry Diseases by J L VegadDocument144 pagesA Color Atlas of Poultry Diseases by J L VegadId DyNo ratings yet
- Rhenogran Short Fiber Reinforcement For Rubber A4!04!2017Document6 pagesRhenogran Short Fiber Reinforcement For Rubber A4!04!2017vlabat2017No ratings yet
- 16-31 Maret 2021Document23 pages16-31 Maret 2021Medika AntapaniNo ratings yet
- Department of Education: Republic of The PhilippinesDocument5 pagesDepartment of Education: Republic of The PhilippinesMaria Madonna BucaoNo ratings yet
- Part I. Assessment On Emotional Quotient (Modified: Daniel Goleman EQ Test, 1995)Document4 pagesPart I. Assessment On Emotional Quotient (Modified: Daniel Goleman EQ Test, 1995)Jennalyn AdatoNo ratings yet
- Exemplification EssayDocument3 pagesExemplification EssayEmily LudemannNo ratings yet
- Immokalee Residents Demand Justice at Vigil For Single Father Killed by Collier Deputy - Naples Daily News 03022021Document2 pagesImmokalee Residents Demand Justice at Vigil For Single Father Killed by Collier Deputy - Naples Daily News 03022021Omar Rodriguez OrtizNo ratings yet
- ECLOS-Difference Course OutlineDocument3 pagesECLOS-Difference Course OutlineJosé Manuel Valdez RevillaNo ratings yet
- Hypertension FamcoDocument42 pagesHypertension FamcoMusleh Al MusalhiNo ratings yet
- Nabl 400Document669 pagesNabl 400Apex IndiaNo ratings yet
- Hellagrolip Nitrogenous Brochure enDocument2 pagesHellagrolip Nitrogenous Brochure enBashir A. SialNo ratings yet
- Bill of Lading / Guía de Carga: Corpus Christi, TX 78466 Tel. (361) 814-7867 TAX ID:81-3031542 TMF Ticket#Document1 pageBill of Lading / Guía de Carga: Corpus Christi, TX 78466 Tel. (361) 814-7867 TAX ID:81-3031542 TMF Ticket#Victor Ingmar abisaiNo ratings yet
- Project Report On Garment BusinessDocument2 pagesProject Report On Garment BusinessAnupNo ratings yet
- Science Meets Spirituality EbookDocument78 pagesScience Meets Spirituality Ebookscriberone100% (1)
- Denon DN 2000fDocument67 pagesDenon DN 2000fDimitar SimeonovNo ratings yet
- MTL8000 1-1 IS Isol PDFDocument1 pageMTL8000 1-1 IS Isol PDFapisituNo ratings yet
- Abi Bro4467 State of Market v10Document36 pagesAbi Bro4467 State of Market v10Pradyut TiwariNo ratings yet
- ITT American Electric Ultra Flood Series 277 & 278 Spec Sheet 2-80Document6 pagesITT American Electric Ultra Flood Series 277 & 278 Spec Sheet 2-80Alan MastersNo ratings yet
- ABG QuizDocument3 pagesABG QuizMelchor Felipe SalvosaNo ratings yet
- Solution Manual For Mathematical Applications For The Management Life and Social Sciences 12th Edition Ronald J Harshbarger James J ReynoldsDocument36 pagesSolution Manual For Mathematical Applications For The Management Life and Social Sciences 12th Edition Ronald J Harshbarger James J Reynoldsdiclinicauroravsl9100% (53)
- Pharmacology of AntidepressantsDocument28 pagesPharmacology of Antidepressantsحيدر كريم سعيد حمزهNo ratings yet
- Alternador Emerson LSA46 2Document12 pagesAlternador Emerson LSA46 2Alvaro Jaime MartínNo ratings yet
- Quick PharmaDocument4 pagesQuick Pharmahva.terrenceavillaNo ratings yet