Professional Documents
Culture Documents
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Uploaded by
yuuahraCopyright:
Available Formats
You might also like
- Marking Scheme For Core Worksheet 1 - Chapter 1Document4 pagesMarking Scheme For Core Worksheet 1 - Chapter 1Anonymous fFKqcY33% (3)
- Farmakokinetika Tugas Soal PPT EkstravaskulerDocument17 pagesFarmakokinetika Tugas Soal PPT Ekstravaskulertaka oneokrock18No ratings yet
- Ujian Akhir Semester Genap Tahun Akademik 2016/2017 Prodi S1 Teknik Perminyakan (Reguler) Sekolah Tinggi Teknologi Minyak Dan Gas Bumi BalikpapanDocument5 pagesUjian Akhir Semester Genap Tahun Akademik 2016/2017 Prodi S1 Teknik Perminyakan (Reguler) Sekolah Tinggi Teknologi Minyak Dan Gas Bumi BalikpapanFernando AbasNo ratings yet
- EKSTRAKSIDocument7 pagesEKSTRAKSISeyzhea Nimas HapsariNo ratings yet
- #08 Lampiran 8 HSS Nakayasu Distribusi Hujan 12 JamDocument11 pages#08 Lampiran 8 HSS Nakayasu Distribusi Hujan 12 Jamchandra adriawanNo ratings yet
- Ejercicio PMS - 2022Document20 pagesEjercicio PMS - 2022LUIS ENRIQUE FRANCO EGUEZNo ratings yet
- Data Distribusi PartikelDocument3 pagesData Distribusi PartikelMovic Clips & ClipsNo ratings yet
- Rumus Persamaan Freundlich Q K C Log Q Log K + 1/n Log CDocument5 pagesRumus Persamaan Freundlich Q K C Log Q Log K + 1/n Log Cmega chaniaNo ratings yet
- Praktikum Biofarmasetika: Data Penetrasi TransdermalDocument6 pagesPraktikum Biofarmasetika: Data Penetrasi TransdermalCindy Riana Putri FebrianiNo ratings yet
- Lembar Perhitungan ABSDocument12 pagesLembar Perhitungan ABSNearya SondangNo ratings yet
- CHAPTER 1 Gen ChemDocument4 pagesCHAPTER 1 Gen ChemYaniiNo ratings yet
- Biophysics - Raport 2Document11 pagesBiophysics - Raport 2276242No ratings yet
- Lembar Perhitungan 1. Menghitung Fraksi Ruang KosongDocument8 pagesLembar Perhitungan 1. Menghitung Fraksi Ruang KosongFikri Risang AdiNo ratings yet
- Book 1Document6 pagesBook 1Yuki KurniawanNo ratings yet
- Jawab:: 2co K MDocument6 pagesJawab:: 2co K ManisaNo ratings yet
- Polimer ReviziiiDocument6 pagesPolimer ReviziiianisaNo ratings yet
- Perfil de Presion: Buildup TestDocument15 pagesPerfil de Presion: Buildup TestTaylor Santiago Salinas Del PezoNo ratings yet
- Hydro Mechanical Erection Estimating 2021Document45 pagesHydro Mechanical Erection Estimating 2021samNo ratings yet
- BiofarmasetikaDocument14 pagesBiofarmasetikagjosNo ratings yet
- Ml/s X 0.1 N 0.0538 N: 0 NaohDocument2 pagesMl/s X 0.1 N 0.0538 N: 0 NaohNabilaFatinKamilasariNo ratings yet
- Bufe Aaron A. SuperheaterDocument5 pagesBufe Aaron A. SuperheaterArabia, Elmo C.No ratings yet
- Baiq Gita AuliaDocument6 pagesBaiq Gita AuliaSUHARTINo ratings yet
- Tabel Hidrograf Inflow: Baiq Gita Aulia F1A019030Document6 pagesTabel Hidrograf Inflow: Baiq Gita Aulia F1A019030SUHARTINo ratings yet
- 182 - Lemper - 4 KamisDocument14 pages182 - Lemper - 4 KamisRiza Shinta RNo ratings yet
- 181 Lemper 4 KamisDocument13 pages181 Lemper 4 KamisRiza Shinta RNo ratings yet
- 647.096 ° 0.54883664 2. Calcula La Presion Reducida PRDocument5 pages647.096 ° 0.54883664 2. Calcula La Presion Reducida PRDaniel Almazan SantillanNo ratings yet
- N W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenDocument15 pagesN W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenRiza Shinta RNo ratings yet
- Lab RebortDocument5 pagesLab RebortAmr SewilamNo ratings yet
- KFHC TPB Reguler ZfactorDocument9 pagesKFHC TPB Reguler ZfactorIlhamRifaldiNo ratings yet
- Lfs2-Calculos 4Document7 pagesLfs2-Calculos 4ALONDRA GUADALUPE GUTIERREZ ROMONo ratings yet
- Bab V Hasil Pengamatan Dan PembahasanDocument11 pagesBab V Hasil Pengamatan Dan PembahasanMuhammad AlmadhanyNo ratings yet
- Data and Results Computations Expt5Document6 pagesData and Results Computations Expt5John Pierre JerusalemNo ratings yet
- PDF 11-0078 Camg (Co3) 2 Dolomite PDF 05-0586 Caco3 Calcite, Syn 11.9% MajorDocument3 pagesPDF 11-0078 Camg (Co3) 2 Dolomite PDF 05-0586 Caco3 Calcite, Syn 11.9% MajorsonficyusNo ratings yet
- Student: Victor Pugliese R#: 11492336 Course: Advanced Reservoir Engineering (PETR 5320) Homework #6Document4 pagesStudent: Victor Pugliese R#: 11492336 Course: Advanced Reservoir Engineering (PETR 5320) Homework #6Victor Pugliese ManotasNo ratings yet
- Yuliadi Tugas 2Document16 pagesYuliadi Tugas 2Firman CahyadiNo ratings yet
- AxcelPutraPrakoso XIPA8 09 ISOTERMIKDocument3 pagesAxcelPutraPrakoso XIPA8 09 ISOTERMIKbackupwoiuntukngebackupNo ratings yet
- Esterification of EthanolDocument15 pagesEsterification of EthanolSadia HasanNo ratings yet
- Fet KovichDocument4 pagesFet KovichStevenGuamanNo ratings yet
- Tabel Csa Otw !!! New KapalDocument16 pagesTabel Csa Otw !!! New KapalPutri Fajar PuspitaNo ratings yet
- Hitungan FIX BANGETDocument5 pagesHitungan FIX BANGET18098C Rekianarsyi A.No ratings yet
- Data Hasil Pengamatan Ikatan ProteinDocument8 pagesData Hasil Pengamatan Ikatan ProteinDimas Nur HidayatNo ratings yet
- Carga-Momento Mampostería ReforzadaDocument30 pagesCarga-Momento Mampostería ReforzadaPamelaCalderónNo ratings yet
- HSS Itb-2Document116 pagesHSS Itb-2Aidil AuliaNo ratings yet
- Test Bank For Absolute C 5th Edition Savitch Mock 013283071X 9780132830713Document36 pagesTest Bank For Absolute C 5th Edition Savitch Mock 013283071X 9780132830713johnbellgienbqscjt100% (31)
- Ejercicios de M.s.2.grupDocument26 pagesEjercicios de M.s.2.grupYamel R. Miranda MaguiñaNo ratings yet
- Topic 8: SPC: Mean Value of First Subgroup (Document4 pagesTopic 8: SPC: Mean Value of First Subgroup (Azlie AzizNo ratings yet
- 1Document7 pages1miladrahimianNo ratings yet
- Lampiran 2 Perhitungan Neraca PanasDocument19 pagesLampiran 2 Perhitungan Neraca PanasWali YudinNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- Rikha Khusniyyah - Tugas3 CREDocument6 pagesRikha Khusniyyah - Tugas3 CREMuhammad SupriyadiNo ratings yet
- #09 Lampiran 9 HSS Nakayasu Distribusi Hujan 6 JamDocument11 pages#09 Lampiran 9 HSS Nakayasu Distribusi Hujan 6 Jamchandra adriawanNo ratings yet
- Common Exhaust Gas C C CDocument3 pagesCommon Exhaust Gas C C CKhristel PenoliarNo ratings yet
- Assignment #2 SolutionDocument9 pagesAssignment #2 SolutionSaqib IrshadNo ratings yet
- N W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenDocument10 pagesN W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenRiza Shinta RNo ratings yet
- Diagrama Predominio Especies Velazquez Cardenas KarlaDocument8 pagesDiagrama Predominio Especies Velazquez Cardenas Karla319174896No ratings yet
- Tahanan Kapal 1Document34 pagesTahanan Kapal 1Fadhil Javier FannaNo ratings yet
- Tabel Perencanaan Kuva Sectional Area (Csa) : MasukanDocument10 pagesTabel Perencanaan Kuva Sectional Area (Csa) : MasukanZarkasi ManshurNo ratings yet
- Sustancia A B C T Sat (Oc) : P Sat1 (MMHG) Psat2 (MMHG)Document12 pagesSustancia A B C T Sat (Oc) : P Sat1 (MMHG) Psat2 (MMHG)Yecid Araceli Nina AguirreNo ratings yet
- Determination Planck ConstatntDocument9 pagesDetermination Planck ConstatntnotmcantekNo ratings yet
- Laboratory Exercises in Astronomy: Solutions and AnswersFrom EverandLaboratory Exercises in Astronomy: Solutions and AnswersNo ratings yet
- Farmasi 2015 Kelas A: Teori KosmetologiDocument3 pagesFarmasi 2015 Kelas A: Teori KosmetologiyuuahraNo ratings yet
- Nama Produk: Body Lotion Bentuk Sediaan: Shinzu'i Produsen: PT Bina Karya PrimaDocument2 pagesNama Produk: Body Lotion Bentuk Sediaan: Shinzu'i Produsen: PT Bina Karya PrimayuuahraNo ratings yet
- Octyl SalicylatDocument1 pageOctyl SalicylatyuuahraNo ratings yet
- May RJ. in - PhaDocument1 pageMay RJ. in - PhayuuahraNo ratings yet
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Uploaded by
yuuahraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Bab Iv Hasil Dan Pembahasan 1.1 Hasil 1.1.1 Perhitungan Percobaan Praktikum Waktu (Menit) Absorbansi CP (PPM) LN CP
Uploaded by
yuuahraCopyright:
Available Formats
BAB IV
HASIL DAN PEMBAHASAN
1.1 Hasil
1.1.1 Perhitungan Percobaan Praktikum
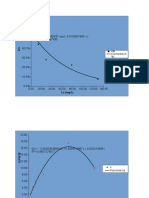
Waktu (menit) Absorbansi Cp (ppm) Ln Cp
2,5 0.503 6,312 1,842
5 0.385 4,871 1,583
10 0.224 2,905 1,066
20 0.077 1,099 0,094
30 0.198 2,583 0,948
60 0.071 1,025 0,024
61 0.692 8,619 2,153
1.1.2 Perhitungan Teoritis
𝟏−𝒆¯ᶯᵏᵀ
Konsentrasi teoritis dihitung menggunakan rumus: Cp = Co [ 𝟏−𝒆¯ᵏᵀ ] e¯ᵏᵗ
Co = 10 ppm
1000 x 1 ml = M x 100 ml = 10 ppm
𝑚𝑙
𝐶𝑙 10
K = 𝑉𝑑 = 𝑚𝑒𝑛𝑖𝑡
100 𝑚𝑙
= 0,1/menit = 6/jam
𝛕 = 20 menit = 0,33 jam
t ½ = 0.693/6 = 0.115 jam
t (jam) 𝜼 Perhitungan Cp Ln Cp
2,5 1 1−𝑒 −1.6.0.33 7,791 2,052969
=0,0416 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.0.0416
60
5 1 1−𝑒 −1.6.0.33 6,066 1,802699
=0,0833 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.0.0833
60
10 1 1−𝑒 −1.6.0.33 3,680 1,302913
=0,1666 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.0.1666
60
20 1 1−𝑒 −1.6.0.33 1,353 0,302324
=0,3333 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.0.3333
60
30 2 1−𝑒 −2.6.0.33 4,188 1,432223
=0,1666 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.0.1666
60
60 3 1−𝑒 −3.6.0.33 1,564 0,447247
=1 Cp = 10 [ 1−𝑒 −6.0.33
] 𝑒 −6.1
60
61 4 1−𝑒 −4.6.0.33 10,482 2,34966
=1,0166 Cp = 10 [ ] 𝑒 −6.1.0166
60 1−𝑒 −6.0.33
1.1.3 Perbandingan Konsentrasi antara Hasil Percobaan dan Pehitungan Teoritis
Cp (ppm)
Waktu (menit)
Hasil percobaan Perhitungan Teoritis
2,5 6.312 7,791
5 4.871 6,066
10 2.905 3,680
20 1.099 1,353
30 2.583 4,188
60 1.025 1,564
61 8.619 10,482
You might also like
- Marking Scheme For Core Worksheet 1 - Chapter 1Document4 pagesMarking Scheme For Core Worksheet 1 - Chapter 1Anonymous fFKqcY33% (3)
- Farmakokinetika Tugas Soal PPT EkstravaskulerDocument17 pagesFarmakokinetika Tugas Soal PPT Ekstravaskulertaka oneokrock18No ratings yet
- Ujian Akhir Semester Genap Tahun Akademik 2016/2017 Prodi S1 Teknik Perminyakan (Reguler) Sekolah Tinggi Teknologi Minyak Dan Gas Bumi BalikpapanDocument5 pagesUjian Akhir Semester Genap Tahun Akademik 2016/2017 Prodi S1 Teknik Perminyakan (Reguler) Sekolah Tinggi Teknologi Minyak Dan Gas Bumi BalikpapanFernando AbasNo ratings yet
- EKSTRAKSIDocument7 pagesEKSTRAKSISeyzhea Nimas HapsariNo ratings yet
- #08 Lampiran 8 HSS Nakayasu Distribusi Hujan 12 JamDocument11 pages#08 Lampiran 8 HSS Nakayasu Distribusi Hujan 12 Jamchandra adriawanNo ratings yet
- Ejercicio PMS - 2022Document20 pagesEjercicio PMS - 2022LUIS ENRIQUE FRANCO EGUEZNo ratings yet
- Data Distribusi PartikelDocument3 pagesData Distribusi PartikelMovic Clips & ClipsNo ratings yet
- Rumus Persamaan Freundlich Q K C Log Q Log K + 1/n Log CDocument5 pagesRumus Persamaan Freundlich Q K C Log Q Log K + 1/n Log Cmega chaniaNo ratings yet
- Praktikum Biofarmasetika: Data Penetrasi TransdermalDocument6 pagesPraktikum Biofarmasetika: Data Penetrasi TransdermalCindy Riana Putri FebrianiNo ratings yet
- Lembar Perhitungan ABSDocument12 pagesLembar Perhitungan ABSNearya SondangNo ratings yet
- CHAPTER 1 Gen ChemDocument4 pagesCHAPTER 1 Gen ChemYaniiNo ratings yet
- Biophysics - Raport 2Document11 pagesBiophysics - Raport 2276242No ratings yet
- Lembar Perhitungan 1. Menghitung Fraksi Ruang KosongDocument8 pagesLembar Perhitungan 1. Menghitung Fraksi Ruang KosongFikri Risang AdiNo ratings yet
- Book 1Document6 pagesBook 1Yuki KurniawanNo ratings yet
- Jawab:: 2co K MDocument6 pagesJawab:: 2co K ManisaNo ratings yet
- Polimer ReviziiiDocument6 pagesPolimer ReviziiianisaNo ratings yet
- Perfil de Presion: Buildup TestDocument15 pagesPerfil de Presion: Buildup TestTaylor Santiago Salinas Del PezoNo ratings yet
- Hydro Mechanical Erection Estimating 2021Document45 pagesHydro Mechanical Erection Estimating 2021samNo ratings yet
- BiofarmasetikaDocument14 pagesBiofarmasetikagjosNo ratings yet
- Ml/s X 0.1 N 0.0538 N: 0 NaohDocument2 pagesMl/s X 0.1 N 0.0538 N: 0 NaohNabilaFatinKamilasariNo ratings yet
- Bufe Aaron A. SuperheaterDocument5 pagesBufe Aaron A. SuperheaterArabia, Elmo C.No ratings yet
- Baiq Gita AuliaDocument6 pagesBaiq Gita AuliaSUHARTINo ratings yet
- Tabel Hidrograf Inflow: Baiq Gita Aulia F1A019030Document6 pagesTabel Hidrograf Inflow: Baiq Gita Aulia F1A019030SUHARTINo ratings yet
- 182 - Lemper - 4 KamisDocument14 pages182 - Lemper - 4 KamisRiza Shinta RNo ratings yet
- 181 Lemper 4 KamisDocument13 pages181 Lemper 4 KamisRiza Shinta RNo ratings yet
- 647.096 ° 0.54883664 2. Calcula La Presion Reducida PRDocument5 pages647.096 ° 0.54883664 2. Calcula La Presion Reducida PRDaniel Almazan SantillanNo ratings yet
- N W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenDocument15 pagesN W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenRiza Shinta RNo ratings yet
- Lab RebortDocument5 pagesLab RebortAmr SewilamNo ratings yet
- KFHC TPB Reguler ZfactorDocument9 pagesKFHC TPB Reguler ZfactorIlhamRifaldiNo ratings yet
- Lfs2-Calculos 4Document7 pagesLfs2-Calculos 4ALONDRA GUADALUPE GUTIERREZ ROMONo ratings yet
- Bab V Hasil Pengamatan Dan PembahasanDocument11 pagesBab V Hasil Pengamatan Dan PembahasanMuhammad AlmadhanyNo ratings yet
- Data and Results Computations Expt5Document6 pagesData and Results Computations Expt5John Pierre JerusalemNo ratings yet
- PDF 11-0078 Camg (Co3) 2 Dolomite PDF 05-0586 Caco3 Calcite, Syn 11.9% MajorDocument3 pagesPDF 11-0078 Camg (Co3) 2 Dolomite PDF 05-0586 Caco3 Calcite, Syn 11.9% MajorsonficyusNo ratings yet
- Student: Victor Pugliese R#: 11492336 Course: Advanced Reservoir Engineering (PETR 5320) Homework #6Document4 pagesStudent: Victor Pugliese R#: 11492336 Course: Advanced Reservoir Engineering (PETR 5320) Homework #6Victor Pugliese ManotasNo ratings yet
- Yuliadi Tugas 2Document16 pagesYuliadi Tugas 2Firman CahyadiNo ratings yet
- AxcelPutraPrakoso XIPA8 09 ISOTERMIKDocument3 pagesAxcelPutraPrakoso XIPA8 09 ISOTERMIKbackupwoiuntukngebackupNo ratings yet
- Esterification of EthanolDocument15 pagesEsterification of EthanolSadia HasanNo ratings yet
- Fet KovichDocument4 pagesFet KovichStevenGuamanNo ratings yet
- Tabel Csa Otw !!! New KapalDocument16 pagesTabel Csa Otw !!! New KapalPutri Fajar PuspitaNo ratings yet
- Hitungan FIX BANGETDocument5 pagesHitungan FIX BANGET18098C Rekianarsyi A.No ratings yet
- Data Hasil Pengamatan Ikatan ProteinDocument8 pagesData Hasil Pengamatan Ikatan ProteinDimas Nur HidayatNo ratings yet
- Carga-Momento Mampostería ReforzadaDocument30 pagesCarga-Momento Mampostería ReforzadaPamelaCalderónNo ratings yet
- HSS Itb-2Document116 pagesHSS Itb-2Aidil AuliaNo ratings yet
- Test Bank For Absolute C 5th Edition Savitch Mock 013283071X 9780132830713Document36 pagesTest Bank For Absolute C 5th Edition Savitch Mock 013283071X 9780132830713johnbellgienbqscjt100% (31)
- Ejercicios de M.s.2.grupDocument26 pagesEjercicios de M.s.2.grupYamel R. Miranda MaguiñaNo ratings yet
- Topic 8: SPC: Mean Value of First Subgroup (Document4 pagesTopic 8: SPC: Mean Value of First Subgroup (Azlie AzizNo ratings yet
- 1Document7 pages1miladrahimianNo ratings yet
- Lampiran 2 Perhitungan Neraca PanasDocument19 pagesLampiran 2 Perhitungan Neraca PanasWali YudinNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- Rikha Khusniyyah - Tugas3 CREDocument6 pagesRikha Khusniyyah - Tugas3 CREMuhammad SupriyadiNo ratings yet
- #09 Lampiran 9 HSS Nakayasu Distribusi Hujan 6 JamDocument11 pages#09 Lampiran 9 HSS Nakayasu Distribusi Hujan 6 Jamchandra adriawanNo ratings yet
- Common Exhaust Gas C C CDocument3 pagesCommon Exhaust Gas C C CKhristel PenoliarNo ratings yet
- Assignment #2 SolutionDocument9 pagesAssignment #2 SolutionSaqib IrshadNo ratings yet
- N W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenDocument10 pagesN W BM Naoh × V × 98 % W Naoh Gram Mol × ×98 %: Lembar Perhitungan ReagenRiza Shinta RNo ratings yet
- Diagrama Predominio Especies Velazquez Cardenas KarlaDocument8 pagesDiagrama Predominio Especies Velazquez Cardenas Karla319174896No ratings yet
- Tahanan Kapal 1Document34 pagesTahanan Kapal 1Fadhil Javier FannaNo ratings yet
- Tabel Perencanaan Kuva Sectional Area (Csa) : MasukanDocument10 pagesTabel Perencanaan Kuva Sectional Area (Csa) : MasukanZarkasi ManshurNo ratings yet
- Sustancia A B C T Sat (Oc) : P Sat1 (MMHG) Psat2 (MMHG)Document12 pagesSustancia A B C T Sat (Oc) : P Sat1 (MMHG) Psat2 (MMHG)Yecid Araceli Nina AguirreNo ratings yet
- Determination Planck ConstatntDocument9 pagesDetermination Planck ConstatntnotmcantekNo ratings yet
- Laboratory Exercises in Astronomy: Solutions and AnswersFrom EverandLaboratory Exercises in Astronomy: Solutions and AnswersNo ratings yet
- Farmasi 2015 Kelas A: Teori KosmetologiDocument3 pagesFarmasi 2015 Kelas A: Teori KosmetologiyuuahraNo ratings yet
- Nama Produk: Body Lotion Bentuk Sediaan: Shinzu'i Produsen: PT Bina Karya PrimaDocument2 pagesNama Produk: Body Lotion Bentuk Sediaan: Shinzu'i Produsen: PT Bina Karya PrimayuuahraNo ratings yet
- Octyl SalicylatDocument1 pageOctyl SalicylatyuuahraNo ratings yet
- May RJ. in - PhaDocument1 pageMay RJ. in - PhayuuahraNo ratings yet