Professional Documents
Culture Documents
Yellow Adjective: Blue Characterized (Noun)
Yellow Adjective: Blue Characterized (Noun)
Uploaded by
Victor Aristizabal0 ratings0% found this document useful (0 votes)
18 views1 pageChemists study matter which has mass and takes up space. Classical thermodynamics examines the macroscopic aspects of matter, dealing with the properties of large groups of microscopic particles like molecules, atoms, and ions. A thermodynamic system is defined as any three-dimensional space that is the focus, usually separated from its surroundings by a boundary like a physical surface or imaginary border. The system and its characteristics can change over time.
Original Description:
English text with verbs
Original Title
English
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentChemists study matter which has mass and takes up space. Classical thermodynamics examines the macroscopic aspects of matter, dealing with the properties of large groups of microscopic particles like molecules, atoms, and ions. A thermodynamic system is defined as any three-dimensional space that is the focus, usually separated from its surroundings by a boundary like a physical surface or imaginary border. The system and its characteristics can change over time.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
18 views1 pageYellow Adjective: Blue Characterized (Noun)
Yellow Adjective: Blue Characterized (Noun)
Uploaded by
Victor AristizabalChemists study matter which has mass and takes up space. Classical thermodynamics examines the macroscopic aspects of matter, dealing with the properties of large groups of microscopic particles like molecules, atoms, and ions. A thermodynamic system is defined as any three-dimensional space that is the focus, usually separated from its surroundings by a boundary like a physical surface or imaginary border. The system and its characteristics can change over time.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
Chemists are interested in systems containing matter—that which has
mass and occupies physical space. Classical thermodynamics looks at
macroscopic aspects of matter. It deals with the properties of aggregates
of vast numbers of microscopic particles (molecules, atoms, and ions).
The macroscopic viewpoint, in fact, treats matter as a continuous
material medium rather than as the collection of discrete microscopic
particles we know are actually present. Although this book is an
exposition of classical thermodynamics, at times it will point out
connections between macroscopic properties and molecular structure
and behavior. A thermodynamic system is any three-dimensional region
of physical space on which we wish to focus our attention. Usually we
consider only one system at a time and call it simply “the system.” The
rest of the physical universe constitutes the surroundings of the system.
The boundary is the closed three-dimensional surface that encloses the
system and separates it from the surroundings. The boundary may (and
usually does) coincide with real physical surfaces: the interface between
two phases, the inner or outer surface of the wall of a flask or other
vessel, and so on. Alternatively, part or all of the boundary may be an
imagined intangible surface in space, unrelated to any physical
structure. The size and shape of the system, as defined by its boundary,
may change in time. In short, our choice of the three-dimensional region
that constitutes the system is arbitrary—but it is essential that we know
exactly what this choice is.
Yellow = Adjective
Blue = Characterized (Noun)
You might also like
- BS 8002 (2015) Code of Practice For Retaining StructuresDocument104 pagesBS 8002 (2015) Code of Practice For Retaining Structuresrichardhenrylamb100% (3)
- Astm B633Document6 pagesAstm B633Ho Bach100% (2)
- Hidden ConnectionsDocument15 pagesHidden ConnectionsCristina Craciun100% (2)
- Single Replacement Reaction Worksheet KeyDocument1 pageSingle Replacement Reaction Worksheet KeyTricia Dionela100% (1)
- 1 - 7-PDF - Thermodynamics (Red Book) 2Document1 page1 - 7-PDF - Thermodynamics (Red Book) 2PranayNo ratings yet
- PrefaceNetwork of ScienceDocument4 pagesPrefaceNetwork of ScienceAkef AfanehNo ratings yet
- The Macroscopic Quantum Effect in Nonlinear Oscillating Systems: A Possible Bridge Between Classical and Quantum Physics Danil Doubochinski and Jonathan TennenbaumDocument16 pagesThe Macroscopic Quantum Effect in Nonlinear Oscillating Systems: A Possible Bridge Between Classical and Quantum Physics Danil Doubochinski and Jonathan Tennenbaumwebmaster8472No ratings yet
- Branches of ScienceDocument4 pagesBranches of ScienceJohn Nathaniel LopezNo ratings yet
- Systems Ecology BookDocument66 pagesSystems Ecology BookArturo AndresNo ratings yet
- Main 170713055801Document151 pagesMain 170713055801Zain RahimNo ratings yet
- 1PART 1 Classical Thermodynamics ReviewDocument204 pages1PART 1 Classical Thermodynamics ReviewBobNo ratings yet
- Intro To Engineering PhysicsDocument11 pagesIntro To Engineering PhysicsBrandon DaseNo ratings yet
- Intro To Engineering PhysicsDocument11 pagesIntro To Engineering PhysicsBrandon DaseNo ratings yet
- What Is Statistical Mechanics?: Roman FriggDocument27 pagesWhat Is Statistical Mechanics?: Roman FriggDiego SaraviaNo ratings yet
- What Is Statistical MechanicsDocument27 pagesWhat Is Statistical MechanicsGilbert FranklinNo ratings yet
- Importance of ChemistryDocument13 pagesImportance of ChemistryRoksana IslamNo ratings yet
- PhysicsDocument1 pagePhysicsVB TwinsNo ratings yet
- Physics Is TheDocument16 pagesPhysics Is TheJonathan CaronNo ratings yet
- ch05 PDFDocument8 pagesch05 PDFFAIQNo ratings yet
- Most Important Science Principles Part IDocument1 pageMost Important Science Principles Part IvardkakNo ratings yet
- Emergence: From The Beginning': Intro To Nanotechnology Boworred From Foothill CollegesDocument50 pagesEmergence: From The Beginning': Intro To Nanotechnology Boworred From Foothill CollegesEdwin Guerrero CRNo ratings yet
- Physics and Scopes of Physics Physics Is The Major Science Dealing With The Fundamental Constituents of The UniverseDocument2 pagesPhysics and Scopes of Physics Physics Is The Major Science Dealing With The Fundamental Constituents of The UniverseernaNo ratings yet
- Bunge 1962 Cosmology MagicDocument27 pagesBunge 1962 Cosmology MagicMarcelo GalvezNo ratings yet
- Lecture 1 Introduction To Physical ChemistryDocument7 pagesLecture 1 Introduction To Physical ChemistryScrappy WellNo ratings yet
- Complex Systems A SurveyDocument10 pagesComplex Systems A SurveyRafael Soares PintoNo ratings yet
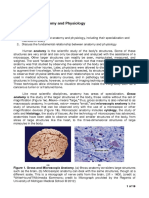
- Module 1A - Overview of Anatomy and PhysiologyDocument19 pagesModule 1A - Overview of Anatomy and PhysiologyRafael SumayaNo ratings yet
- Chemistry: MatterDocument13 pagesChemistry: MatterPsalm Beato DichosoNo ratings yet
- Edgar Morin, Restricted Complexity, General ComplexityDocument25 pagesEdgar Morin, Restricted Complexity, General ComplexityJuan GerardoNo ratings yet
- Anatomy and PhysiologyDocument18 pagesAnatomy and PhysiologyKatrina ParbaNo ratings yet
- F. Reif - Statistical Physics - Chapter 1 PDFDocument51 pagesF. Reif - Statistical Physics - Chapter 1 PDFIndrawati WilujengNo ratings yet
- Auxiliary Sciences of PhysicsDocument7 pagesAuxiliary Sciences of PhysicsScribdTranslationsNo ratings yet
- Fields of Study of PhysicsDocument35 pagesFields of Study of PhysicsScribdTranslationsNo ratings yet
- Introduction To Thermodynamics With ApplicationsDocument271 pagesIntroduction To Thermodynamics With Applicationsdiyar_elissa1575No ratings yet
- Ast1 BacotoDocument1 pageAst1 BacotoMarchien BacotoNo ratings yet
- Human AnatomyDocument4 pagesHuman AnatomyaprilNo ratings yet
- Physics Class XI SCDocument13 pagesPhysics Class XI SCAyush Abhinandan RathNo ratings yet
- Universal Dynamics, A Unified Theory of Complex Systems. Emergence, Life and DeathDocument57 pagesUniversal Dynamics, A Unified Theory of Complex Systems. Emergence, Life and Deathtestonly261No ratings yet
- Formulations of Classical Mechanics: PhysicsDocument18 pagesFormulations of Classical Mechanics: PhysicsArslan AhmadNo ratings yet
- LBJW, Efblje, ADocument8 pagesLBJW, Efblje, Ahaseje4827No ratings yet
- Earth Science or Geoscience Is A Widely Embraced Term For TheDocument7 pagesEarth Science or Geoscience Is A Widely Embraced Term For TheJay-b magandamNo ratings yet
- Science PhysicsDocument4 pagesScience PhysicsMarie Elaine B. CaparasNo ratings yet
- The Holographic Concept of Reality - Richard Alan Miller, Burt Webb & Darden DicksonDocument15 pagesThe Holographic Concept of Reality - Richard Alan Miller, Burt Webb & Darden DicksonSamir Al-HamedNo ratings yet
- Significant Sci Theories1Document4 pagesSignificant Sci Theories1Luis VelazquezNo ratings yet
- Summary of Bertalanffy's BookDocument4 pagesSummary of Bertalanffy's BookScribdTranslationsNo ratings yet
- Lesson 3 Outlining Performance TaskDocument2 pagesLesson 3 Outlining Performance Taskaudraerapha1No ratings yet
- Physics and Its BranchesDocument6 pagesPhysics and Its Branchesphysics_freak100% (3)
- 1.1 Continuum Theory: KnownDocument2 pages1.1 Continuum Theory: KnownJason OliverNo ratings yet
- Thermodynamics, and Statistical Mechanics: Internal Energy Temperature Energy Energy State Absolute ZeroDocument3 pagesThermodynamics, and Statistical Mechanics: Internal Energy Temperature Energy Energy State Absolute Zeronikita bajpaiNo ratings yet
- Entropy: On Thermodynamics, Entropy and Evolution of Biological Systems: What Is Life From A Physical Chemist's ViewpointDocument12 pagesEntropy: On Thermodynamics, Entropy and Evolution of Biological Systems: What Is Life From A Physical Chemist's ViewpointPranav NakhateNo ratings yet
- ChemistryDocument1 pageChemistryzinaidamateNo ratings yet
- The Scope of PhysicsDocument23 pagesThe Scope of PhysicsDeane Marc TorioNo ratings yet
- Mechanics, Elasticity, Rheology (Paronia) PhysicsDocument33 pagesMechanics, Elasticity, Rheology (Paronia) Physicssharamaeparonia629No ratings yet
- What Type of Subject Is Physics?Document3 pagesWhat Type of Subject Is Physics?Darry BlanciaNo ratings yet
- Heylighen F-BuildingComplexityDocument16 pagesHeylighen F-BuildingComplexitysharwin sharkNo ratings yet
- A Minimalist Ontology of The Natural World (PDFDrive)Document189 pagesA Minimalist Ontology of The Natural World (PDFDrive)Joseph Hubert Ngon BiramNo ratings yet
- Particle Nature of MatterDocument11 pagesParticle Nature of Matteramora eliNo ratings yet
- Laws in Physics PDFDocument17 pagesLaws in Physics PDFObicho IsaacNo ratings yet
- Chapter 6Document1 pageChapter 6Secre NeroNo ratings yet
- Assigned Terms To Be Studied During Seminarians' Week 2020 - Philo. of NatureDocument3 pagesAssigned Terms To Be Studied During Seminarians' Week 2020 - Philo. of Naturemanu marfzNo ratings yet
- Comparison of BS and BSEN PDFDocument17 pagesComparison of BS and BSEN PDFPrimelift Safety Resources LimitedNo ratings yet
- HVAC LifeDocument8 pagesHVAC LifeNadeem QaisarNo ratings yet
- 2elimination of Selected Heavy Metals From Aqueous Solutions Using Biochar and Bentonite Composite MonolithDocument11 pages2elimination of Selected Heavy Metals From Aqueous Solutions Using Biochar and Bentonite Composite MonolithEyasu WodajoNo ratings yet
- Imperfection in SolidsDocument24 pagesImperfection in SolidsYuda SatriaNo ratings yet
- Specification SmartFenceDocument3 pagesSpecification SmartFenceVimala PonnusamyNo ratings yet
- AISI 1050 Steel, As RolledDocument2 pagesAISI 1050 Steel, As RolledCristobal Gutierrez CarrascoNo ratings yet
- Leachate and Groundwater Quality in Lagos FinalDocument12 pagesLeachate and Groundwater Quality in Lagos FinalLasisi Adedoyin K.S100% (2)
- Cril at 4830Document4 pagesCril at 4830ForeverNo ratings yet
- Norma SAE J 306Document8 pagesNorma SAE J 306jizuNo ratings yet
- Seismic Lateral Pressures in Soils With Cohesion PDFDocument22 pagesSeismic Lateral Pressures in Soils With Cohesion PDFBouraida EL YAMOUNINo ratings yet
- Auger Recombination PDFDocument14 pagesAuger Recombination PDFviperNo ratings yet
- B. Gerard, - Fundamentals of Hardfacing by Fusion Welding, Welding Alloys Group, USADocument35 pagesB. Gerard, - Fundamentals of Hardfacing by Fusion Welding, Welding Alloys Group, USAScienticsPeerNo ratings yet
- Well Test Standards WTS 3.8 Coflexip Hoses: Global ManualDocument9 pagesWell Test Standards WTS 3.8 Coflexip Hoses: Global ManualEmmanuel100% (1)
- Example Compressive Strength TestDocument6 pagesExample Compressive Strength Testmma4801No ratings yet
- QAM2120.040 Environmental Declaration en PDFDocument2 pagesQAM2120.040 Environmental Declaration en PDFosama alabsiNo ratings yet
- Glass Superstructure RefractoryDocument4 pagesGlass Superstructure RefractorySans SenNo ratings yet
- Latent Heat of VaporizationDocument11 pagesLatent Heat of VaporizationEsther Faith GabrielNo ratings yet
- The Variety of Phosphates For Refractory and Technical Applications by The Example of Aluminium PhosphatesDocument5 pagesThe Variety of Phosphates For Refractory and Technical Applications by The Example of Aluminium Phosphatesakanksha kumariNo ratings yet
- Corrosion of Galvanized Steel and Copper in Aqueous EnvironmentsDocument7 pagesCorrosion of Galvanized Steel and Copper in Aqueous EnvironmentsAmrul KaishNo ratings yet
- Bitumen With Salt PDFDocument9 pagesBitumen With Salt PDFbkswain2003No ratings yet
- Googlepreview PDFDocument89 pagesGooglepreview PDFFaris FadliNo ratings yet
- Doc4 El PrincDocument10 pagesDoc4 El PrincKurt CargoNo ratings yet
- Specification For Nickel Base Alloy 625 Plus (UNS N07716) AND ALLOY 725 (UNS N07725) FOR OIL and Gas Drilling and Production EquipmentDocument20 pagesSpecification For Nickel Base Alloy 625 Plus (UNS N07716) AND ALLOY 725 (UNS N07725) FOR OIL and Gas Drilling and Production EquipmentakintundeakinboladeNo ratings yet
- Pas Hink in 2003Document16 pagesPas Hink in 2003石皓瑋No ratings yet
- Guideline On Fabrication (Railway Bridge Code)Document10 pagesGuideline On Fabrication (Railway Bridge Code)amawauceNo ratings yet
- Defects in CrystalsDocument19 pagesDefects in CrystalszeroNo ratings yet