Professional Documents
Culture Documents
Stomach: Structure and Function
Stomach: Structure and Function
Uploaded by
arina aulia0 ratings0% found this document useful (0 votes)
6 views1 pageThis document summarizes the structure and function of the stomach. It discusses:
1) The stomach contains five regions (cardia, fundus, corpus, antrum, pylorus) and has four layers (mucosa, submucosa, muscularis externa, serosa). The mucosa contains gastric glands with chief cells, parietal cells, and mucous neck cells.
2) Parietal cells secrete acid (H+) via proton pumps and transporters that exchange H+ for K+ and Cl-. Stimulation causes a rapid rise in acid and fluid secretion, lowering pH to around 1.
3) The stomach is protected from acid by the gastric
Original Description:
Y
Original Title
first-page-pdf.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes the structure and function of the stomach. It discusses:
1) The stomach contains five regions (cardia, fundus, corpus, antrum, pylorus) and has four layers (mucosa, submucosa, muscularis externa, serosa). The mucosa contains gastric glands with chief cells, parietal cells, and mucous neck cells.
2) Parietal cells secrete acid (H+) via proton pumps and transporters that exchange H+ for K+ and Cl-. Stimulation causes a rapid rise in acid and fluid secretion, lowering pH to around 1.
3) The stomach is protected from acid by the gastric
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
6 views1 pageStomach: Structure and Function
Stomach: Structure and Function
Uploaded by
arina auliaThis document summarizes the structure and function of the stomach. It discusses:
1) The stomach contains five regions (cardia, fundus, corpus, antrum, pylorus) and has four layers (mucosa, submucosa, muscularis externa, serosa). The mucosa contains gastric glands with chief cells, parietal cells, and mucous neck cells.
2) Parietal cells secrete acid (H+) via proton pumps and transporters that exchange H+ for K+ and Cl-. Stimulation causes a rapid rise in acid and fluid secretion, lowering pH to around 1.
3) The stomach is protected from acid by the gastric
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
C H A P T E R 56
Stomach
Luminal peptides and gastric distention stimulate gastrin secre-
STRUCTURE AND FUNCTION tion from G cells and effect histamine release from enterochromaffin-
Kenneth W. Simpson like cells. Gastrin-releasing peptide and acetylcholine are the
primary enteric neurotransmitters regulating gastrin release from
antral G cells (Figure 56-3).
Unstimulated acid secretion in dogs and cats is minimal6-8 (e.g.,
Functional Anatomy
dogs <0.04 mmol/kg0.75/h) and the unstimulated H+/K+-adenosine
The stomach acts as a reservoir to control the size and rate of passage triphosphatase (ATPase), “the proton pump,” is localized within
of ingesta into the small intestine, to initiate the digestion of protein tubulovesicles in the cytoplasm of parietal cells.4,9 The stimulated
and fat, and to facilitate the absorption of vitamins and minerals H+/K+-ATPase and KCl transporters are incorporated into the pari-
(see Chapter 1) etal cell canalicular membrane and hydrogen ions (derived from the
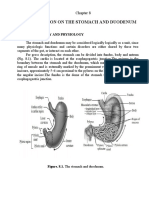
The stomach is composed of five anatomic regions: cardia, ionization of water within the parietal cells) are transported into the
fundus, corpus, antrum, and pylorus (Figure 56-1). The fundus and gastric lumen in exchange for K+ by H+/K+-ATPase. Potassium and
body expand greatly to accommodate ingesta and to regulate the chloride transporters in the canalicular membrane enable luminal
emptying of liquids. The antrum grinds food into smaller particles transfer of potassium (for recycling via H+/K+-ATPase) and chloride.
(<2 mm) that are sieved into the duodenum. The gastroesophageal Hydroxide combines with CO2, catalyzed by carbonic anhydrase, to
sphincter prevents reflux of gastric fluid into the esophagus, and the form HCO3−, which diffuses into the blood giving rise to the “alka-
pyloric sphincter controls emptying into the small intestine. line tide” phenomenon. Recent studies have expanded our knowl-
The gastric wall contains a mucosa, submucosa, muscularis edge of ion transport in the parietal cell by identifying KCNQ1 as
externa, and serosa (see Figures 56-2 and 1-3, B). The mucosa has the primary channel responsible for K+ recycling and establishing
a superficial epithelium, gastric glands, and an innermost layer of the contribution of CFTR (cystic fibrosis transmembrane regulator)
smooth muscle (the muscularis mucosa), with fine structure and and SLC26A9 to the process of chloride secretion.5 Figure 56-4
function varying depending on the gastric region. The mucosa in shows a schematic of ion transport in the parietal cell incorporating
the cardia and pylorus is thinner and less glandular than in the these findings. Stimulation results in a rapid increase in fluid and
fundus and body. The mucosa of the body contains mucous neck hydrogen ion secretion, with pH rapidly declining to around pH 1.
cells (producing mucous, pepsinogen A, and gastric lipase), parietal The concentrations of K+ (10 to 20 mmol/L) and Cl− (approxi-
cells (producing H+, pepsinogen A, and intrinsic factor), and chief mately 120 to 160 mmol/L) in gastric juice are higher than in
cells (producing pepsinogen A) (Figure 56-2).1-4 A variety of neu- plasma.
roendocrine cells are involved in the regulation of acid secretion The stomach is protected from gastric acid injury by a functional
and are interspersed between the glands. The predominant cells are unit known as the gastric mucosal barrier.10,11 The gastric mucosal
enterochromaffin-like and somatostatin-producing cells in the barrier comprises tightly opposed epithelial cells coated with a layer
fundus, and gastrin and somatostatin-producing cells in the antrum of bicarbonate-rich mucus and an abundant mucosal blood supply
(see Figure 56-2). Localized small aggregates of lymphoid tissues are that delivers bicarbonate, oxygen, and nutrients. Local production
frequently observed at the base of the gastric glands. A rich network of prostaglandin (PGE2) is important in modulating blood flow,
of blood vessels, lymphatics, and nerves weaves between the gastric bicarbonate secretion, and epithelial cell renewal. When damage
glands. Beneath the submucosa are two layers of smooth muscle occurs, epithelial cells rapidly migrate over superficial mucosal
(circular and longitudinal) that run perpendicular to one another. defects aided by the local production of growth factors such as epi-
The serosa is the outermost layer. dermal growth factor.
Regulation of Acid Secretion Evaluating Gastric Secretory Function
Physiologically, luminal peptides and gastric distention are the Gastric secretory testing is primarily performed in patients with
primary stimuli for H+ secretion from parietal cells. Pharmacologi- esophagitis, gastrointestinal (GI) ulceration, mucosal hypertrophy,
cally, parietal cell acid secretion is regulated by endocrine (gastrin), and in patients suspected of having acid hypersecretion.
neurocrine (acetylcholine), and paracrine (histamine) mecha- As a starting point, fasting gastric pH and serum gastrin can be
nisms.4,5 Somatostatin released in response to gastric pH levels measured to determine if acid hypersecretion is likely. Ideally, anti-
below 3 provides negative feedback and decreases gastrin, histamine, secretory therapy should be discontinued for 7 days prior to testing,12
and acid secretion. and renal and hepatic function should be monitored as these may
606
You might also like
- ANAPHY Lec Session #21 - SAS (Agdana, Nicole Ken)Document8 pagesANAPHY Lec Session #21 - SAS (Agdana, Nicole Ken)Nicole Ken AgdanaNo ratings yet
- Gastrointestinal Anatomy and Physiology: The EssentialsFrom EverandGastrointestinal Anatomy and Physiology: The EssentialsJohn F. ReinusNo ratings yet
- Digestive System - 1: Learning ObjectivesDocument7 pagesDigestive System - 1: Learning ObjectivesMaggieLockeNo ratings yet
- Р.8.OPERATION ON THE STOMATH AND DUODENUMDocument47 pagesР.8.OPERATION ON THE STOMATH AND DUODENUMMahmood SalahNo ratings yet
- Git HormonesDocument17 pagesGit HormonesAniket MittalNo ratings yet
- Physiology of The PancreasDocument4 pagesPhysiology of The PancreasKetan PatelNo ratings yet
- Mechanism of Gastric SecretionDocument11 pagesMechanism of Gastric Secretionmulya ningsihNo ratings yet
- Lecture 10: Gastric Secretion & Its Regulation: Functions of StomachDocument8 pagesLecture 10: Gastric Secretion & Its Regulation: Functions of StomachBoody KhalilNo ratings yet
- The Physiology of The GIT and The Liver QuestionsDocument44 pagesThe Physiology of The GIT and The Liver QuestionsDonkeyManNo ratings yet
- Gastric AcidDocument5 pagesGastric Acidbrian3442No ratings yet
- Gastrin From Wikipedia, The Free EncyclopediaDocument23 pagesGastrin From Wikipedia, The Free EncyclopediaVincent Lau Bi ShengNo ratings yet
- Farko Cerna UTS + TugasDocument357 pagesFarko Cerna UTS + TugasAnnisa N RahmayantiNo ratings yet
- Gastric Juice RegulationDocument22 pagesGastric Juice Regulationjanuian75% (4)
- Esophages and Stomach ...........Document65 pagesEsophages and Stomach ...........Gebrie DinkayehuNo ratings yet
- Salivary, Gastric + PaDocument34 pagesSalivary, Gastric + PaerisericssonNo ratings yet
- Gastrointestinal PhysiologyDocument6 pagesGastrointestinal PhysiologyPedro RodriguezNo ratings yet
- The GITDocument5 pagesThe GITantoonieNo ratings yet
- Gastricacidand Digestivephysiology: Philip T. Ramsay,, Aaron CarrDocument6 pagesGastricacidand Digestivephysiology: Philip T. Ramsay,, Aaron CarryorleNo ratings yet
- Lecture-5 Hormonal Regulation of Intestinal MotilityDocument4 pagesLecture-5 Hormonal Regulation of Intestinal Motilityمرتضى حسين عبدNo ratings yet
- Peptic Ulcers: PHRM 304Document24 pagesPeptic Ulcers: PHRM 304Apurba Sarker Apu100% (1)
- An Amino Acid Transporter Involved in Gastric Acid SecretionDocument11 pagesAn Amino Acid Transporter Involved in Gastric Acid SecretionAhmad FikriNo ratings yet
- Digestive/Endocrine Answer KeyDocument10 pagesDigestive/Endocrine Answer KeykmulqsNo ratings yet
- The Gastric Phase of The Integrated Response To A MealDocument12 pagesThe Gastric Phase of The Integrated Response To A MealpuchioNo ratings yet
- GIT - Lecture 1-2 UpdatedDocument57 pagesGIT - Lecture 1-2 UpdatedPushpa Paul 2021665649No ratings yet
- Digestive SystemDocument117 pagesDigestive SystemKBDNo ratings yet
- Digestive System - MefloresDocument117 pagesDigestive System - MefloresJohnMichaelDominguezNo ratings yet
- Gastrointestinal System - 2Document35 pagesGastrointestinal System - 2adiarthagriadhiNo ratings yet
- Git Physiology FinalDocument14 pagesGit Physiology FinalVondNo ratings yet
- 6MD GI EnglishDocument86 pages6MD GI EnglishAkachukwu ObunikeNo ratings yet
- Abdominal Surgery All in OneDocument50 pagesAbdominal Surgery All in OneAnne ChoyNo ratings yet
- Acid SecretionpptDocument50 pagesAcid Secretionpptdrkimi ralte2No ratings yet
- Anatomy Digestive SystemDocument57 pagesAnatomy Digestive SystempabitraNo ratings yet
- GIDocument7 pagesGIiheart musicNo ratings yet
- Git Lecture 3Document30 pagesGit Lecture 3sandhiya.peetamberNo ratings yet
- PSG 252 Lecture 3 The StomachDocument5 pagesPSG 252 Lecture 3 The StomachMichael TobilobaNo ratings yet
- 1 IntroductionDocument22 pages1 IntroductionirfanNo ratings yet
- Structure Appearance FunctionDocument6 pagesStructure Appearance FunctionKennee Eve CantilNo ratings yet
- DOI V2. Normal Pancreatic Function - RevisedDocument13 pagesDOI V2. Normal Pancreatic Function - RevisedsaniNo ratings yet
- Gastric SecretionDocument50 pagesGastric SecretionYanglem AmarjitNo ratings yet
- Anatomy and Physiology of The StomachDocument7 pagesAnatomy and Physiology of The StomachEmmi Maliza HutagaolNo ratings yet
- Physio 5. Digestive System 5Document17 pagesPhysio 5. Digestive System 5Xena XenaNo ratings yet
- Digestive System (Unit X)Document44 pagesDigestive System (Unit X)zahraNo ratings yet
- 2019-AGA-DDSEP-9-Chapter-2-1557871764749 1 ENFERMEDADES DEL ESTOMAGODocument24 pages2019-AGA-DDSEP-9-Chapter-2-1557871764749 1 ENFERMEDADES DEL ESTOMAGOEmilia GarciaNo ratings yet
- Surgical Anatomy of Pancreas: DR GaneshDocument42 pagesSurgical Anatomy of Pancreas: DR GaneshGanesh MarutinathNo ratings yet
- 13 Human Physiology Gastrointestinal PhysiologyDocument82 pages13 Human Physiology Gastrointestinal PhysiologysuNo ratings yet
- Physiology, Lecture 8, GIT 2 (Stomach) (Slides)Document24 pagesPhysiology, Lecture 8, GIT 2 (Stomach) (Slides)Ali Al-Qudsi100% (2)
- C PDFDocument10 pagesC PDFTaro RahmatiaNo ratings yet
- Stomach: Clinical Clerk: Jarney Dyenn Bito-OnonDocument44 pagesStomach: Clinical Clerk: Jarney Dyenn Bito-OnonLajel S. LachicaNo ratings yet
- Martamala2001 (Bile Reflux)Document7 pagesMartamala2001 (Bile Reflux)askhaeraniNo ratings yet
- 2 Gastrointestinal System, FK, 2013Document73 pages2 Gastrointestinal System, FK, 2013Cox AbeeNo ratings yet
- 51 Pancreatitis: AnatomyDocument68 pages51 Pancreatitis: AnatomyFairus ZabadiNo ratings yet
- Phys OkDocument7 pagesPhys OkVivek ChaudharyNo ratings yet
- Pathophysiology of DiseaseDocument7 pagesPathophysiology of DiseaseYannah Mae EspineliNo ratings yet
- By124l Case Study 2Document2 pagesBy124l Case Study 2ashlyn granthamNo ratings yet
- Gastrointestinal System, FK, 2013Document72 pagesGastrointestinal System, FK, 2013adiarthagriadhiNo ratings yet
- Regulation of GIT ProcessesDocument16 pagesRegulation of GIT ProcessesMuneeb Ur RehmanNo ratings yet
- Git PharmacoDocument10 pagesGit Pharmacomaxwell amponsahNo ratings yet
- Salivary Composition and RegulationDocument36 pagesSalivary Composition and Regulationanaya84No ratings yet
- Colostomy CareDocument6 pagesColostomy CareLoren Sales ReyesNo ratings yet
- Common Symptoms of Gastrointestinal and Abdominal DiseaseDocument34 pagesCommon Symptoms of Gastrointestinal and Abdominal DiseaseMalueth AnguiNo ratings yet
- Open CholecystectomyDocument7 pagesOpen CholecystectomyMonique CastroNo ratings yet
- Gallstones: Mitchell Conn Educational GoalsDocument8 pagesGallstones: Mitchell Conn Educational GoalsIan Evan LeeNo ratings yet
- Images: How To Draw Human Digestive System Step by ... - YoutubeDocument1 pageImages: How To Draw Human Digestive System Step by ... - YoutubePratham KamaniNo ratings yet
- Gallstones: American College of Gastroenterology Common Gastrointestinal ProblemsDocument2 pagesGallstones: American College of Gastroenterology Common Gastrointestinal Problemsanon100% (1)
- Pediatric Surgery Notes For NursesDocument8 pagesPediatric Surgery Notes For NursesAhmed SamyNo ratings yet
- Probiotics: Microbiology 8 SemesterDocument10 pagesProbiotics: Microbiology 8 SemesterSherdil khanNo ratings yet
- Medic 1Document1 pageMedic 1mainakroy807No ratings yet
- 106 Finals Reviewer Quizlet and NurselabsDocument62 pages106 Finals Reviewer Quizlet and NurselabsCreciabullecerNo ratings yet
- DR Deutsch IASN Convention TalkDocument27 pagesDR Deutsch IASN Convention TalktheresiaaquilaNo ratings yet
- Acute Pancreatitis 2012Document36 pagesAcute Pancreatitis 2012Alex TofanNo ratings yet
- 1-Understanding The Pathophysiology of Intestinal Obstruction, Including The DifferentDocument11 pages1-Understanding The Pathophysiology of Intestinal Obstruction, Including The DifferentフセインNo ratings yet
- Chronic PancreatitisDocument23 pagesChronic PancreatitisAlvin YeeNo ratings yet
- Chronic Constipation: Harvard Medical SchoolDocument7 pagesChronic Constipation: Harvard Medical SchoolrhymenNo ratings yet
- 02 Gastrointestinal SystemDocument31 pages02 Gastrointestinal SystemMSKCNo ratings yet
- Functions of The Gallbladder: Chantal Housset, Yves CHR Etien, Dominique Debray, and Nicolas ChignardDocument29 pagesFunctions of The Gallbladder: Chantal Housset, Yves CHR Etien, Dominique Debray, and Nicolas ChignardiuventasNo ratings yet
- Liver - Disease - Evaluation of Liver Disease in The Pediatric Patient Ian D. D'Agata, MD and William F. Balistreri, MDDocument14 pagesLiver - Disease - Evaluation of Liver Disease in The Pediatric Patient Ian D. D'Agata, MD and William F. Balistreri, MDC MateNo ratings yet
- Class-10 Revision Test - NutritionDocument4 pagesClass-10 Revision Test - NutritionVarshini PeraNo ratings yet
- Rabbit Digestive System PDFDocument2 pagesRabbit Digestive System PDFLehlogonolo LekgwathiNo ratings yet
- 3d NLS Study of Gall Bladder and Gall Ducts ConcretionsDocument3 pages3d NLS Study of Gall Bladder and Gall Ducts ConcretionstestnationNo ratings yet
- Proton Pump Inhibitors PDFDocument4 pagesProton Pump Inhibitors PDFBintoo SharmaNo ratings yet
- Gi Disorders in PregnancyDocument43 pagesGi Disorders in PregnancyDiana MurguiaNo ratings yet
- Lesson Plan 2020 Bio Digestion 1Document6 pagesLesson Plan 2020 Bio Digestion 1Nicketa AndersonNo ratings yet
- Round-The-clock Acid Control of Rabeprazole On Acid Related DisorderDocument8 pagesRound-The-clock Acid Control of Rabeprazole On Acid Related DisorderRabeprazole SodiumNo ratings yet
- Science and Health IvDocument13 pagesScience and Health IvMary Joy Lucob TangbawanNo ratings yet
- History Taking in Medicine and Surgery: Third EditionDocument48 pagesHistory Taking in Medicine and Surgery: Third EditionSapreni agustinaNo ratings yet
- 4th Quarter Biology ReviewerDocument3 pages4th Quarter Biology ReviewerTortelliniNo ratings yet
- Upper Gastrointestinal (GI) BleedingDocument56 pagesUpper Gastrointestinal (GI) BleedingRahul Kumar VermaNo ratings yet
- Necrotizing Pancreatitis - DBarilDocument25 pagesNecrotizing Pancreatitis - DBarilataner1991No ratings yet