Professional Documents
Culture Documents
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Uploaded by
tylerCopyright:
Available Formats
You might also like
- WORKSHEET: Chemical Bonding - Ionic & Covalent! Ionic Bond Covalent BondDocument3 pagesWORKSHEET: Chemical Bonding - Ionic & Covalent! Ionic Bond Covalent BondHaven jethro UrbanoNo ratings yet
- Ionic Bond LabDocument6 pagesIonic Bond LabHuda Wahab0% (1)
- Nmat Reviewer Gen and Ana ChemDocument22 pagesNmat Reviewer Gen and Ana Chemforfuture reviewersNo ratings yet
- HyaFilia Brochure (E) 0804-1Document12 pagesHyaFilia Brochure (E) 0804-1sha100% (1)
- .Ws Ionic Bonding Activity KeyDocument4 pages.Ws Ionic Bonding Activity KeyrajaijahNo ratings yet
- 2020 Unit 5 Notes - TEACHER (Nomenclature)Document11 pages2020 Unit 5 Notes - TEACHER (Nomenclature)Adi ChhNo ratings yet
- Chemistry - Writing Formula and Chemical EquationsDocument18 pagesChemistry - Writing Formula and Chemical EquationsexperiorNo ratings yet
- Naming Flowchart ChemistryDocument1 pageNaming Flowchart ChemistryamyNo ratings yet
- Chapter 2 Stoichiometry Edu NitpdfDocument42 pagesChapter 2 Stoichiometry Edu Nitpdfapi-386303659No ratings yet
- The Periodic TableDocument5 pagesThe Periodic TablePunyathorn KansiriNo ratings yet
- Chemical Formulas-General ChemistryDocument19 pagesChemical Formulas-General Chemistry7assan1300No ratings yet
- ROAD TO MY LEWIS ActivityDocument1 pageROAD TO MY LEWIS ActivityCristy SevillaNo ratings yet
- Types of CompoundsDocument15 pagesTypes of CompoundsJonard PedrosaNo ratings yet
- Nomenclature FlowchartDocument2 pagesNomenclature FlowchartaresamritNo ratings yet
- Formulas and Nomenclature of Ionic and Covalent Compounds: ContentsDocument13 pagesFormulas and Nomenclature of Ionic and Covalent Compounds: Contentsawesome avedNo ratings yet
- Reviewer in ChemistryDocument11 pagesReviewer in Chemistryxian tanNo ratings yet
- Naming Chemical CompoundsDocument44 pagesNaming Chemical Compoundss140917No ratings yet
- Chemistry: Writing Ionic Formulas For CompoundsDocument5 pagesChemistry: Writing Ionic Formulas For CompoundsTiffany GallinaNo ratings yet
- Naming Compounds FlowchartDocument1 pageNaming Compounds Flowchartapi-310503032No ratings yet
- Chemical Formulas KeynoteDocument34 pagesChemical Formulas Keynotewperry42No ratings yet
- Penamaan SenyawaDocument12 pagesPenamaan SenyawaJason Enduro BayuNo ratings yet
- VER2Formulas and Nomenclature of Ionic and Covalent Compounds PDFDocument12 pagesVER2Formulas and Nomenclature of Ionic and Covalent Compounds PDFjamesNo ratings yet
- 02 Polymeric Materials Molecular Lecture 2Document30 pages02 Polymeric Materials Molecular Lecture 2Katrina BucudNo ratings yet
- Ions and MoleculesDocument4 pagesIons and MoleculesITZDUSTY gamerNo ratings yet
- Chemistry TablesDocument3 pagesChemistry Tableswvcs2gz9bbNo ratings yet
- Chapter 1 - 2021-Matter and Nomenclature (Student)Document50 pagesChapter 1 - 2021-Matter and Nomenclature (Student)Micho santuna Putra putraNo ratings yet
- Chem Handout NamingDocument3 pagesChem Handout NamingAlexander DolinNo ratings yet
- Inorganic NomenclatureDocument65 pagesInorganic NomenclatureCalm your MindNo ratings yet
- Malaysha Brunner - Unit 5E Procedures For Naming Ionic CompoundsDocument5 pagesMalaysha Brunner - Unit 5E Procedures For Naming Ionic CompoundsMalaysha BrunnerNo ratings yet
- Ionic BondsDocument3 pagesIonic BondsrajaijahNo ratings yet
- 4 Chemical Formula Naming PDFDocument30 pages4 Chemical Formula Naming PDFDenise SeseNo ratings yet
- 08-Metals and Non-Metals Theory PDFDocument51 pages08-Metals and Non-Metals Theory PDFvikash singh rajpurohitNo ratings yet
- Learning GuideDocument3 pagesLearning GuideLadasha AbuevaNo ratings yet
- (CHEM) Chapter 8 - Periodic TableDocument14 pages(CHEM) Chapter 8 - Periodic TableVijay Kumar NatteyNo ratings yet
- Module 16Document16 pagesModule 16Yeng LopezNo ratings yet
- Chemis - Writing Chemical EquationDocument11 pagesChemis - Writing Chemical EquationZahra PriyonoNo ratings yet
- Naming and Formula Writing of Ionic Compounds: Ms. Ma. Norma Datu Renomeron, MAED Chemistry TeacherDocument83 pagesNaming and Formula Writing of Ionic Compounds: Ms. Ma. Norma Datu Renomeron, MAED Chemistry TeacherDave SallaoNo ratings yet
- Corrosion & Its Control & Its ControlDocument28 pagesCorrosion & Its Control & Its ControlHiren KumarNo ratings yet
- Cpe639 Lecture 4Document73 pagesCpe639 Lecture 4Aisyah Addia AzizanNo ratings yet
- Plating Tutorial 2009Document95 pagesPlating Tutorial 2009gregolgratis100% (2)
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Calculations Involving Balanced Chemical EquationsDocument107 pagesCalculations Involving Balanced Chemical Equationsmain.20002245No ratings yet
- Naming Compounds ChemistryDocument58 pagesNaming Compounds ChemistryRizalyn Padua ReyNo ratings yet
- Common IonsDocument4 pagesCommon Ionssina.aral28No ratings yet
- GC1 Lesson 3 Atoms Molecules and Ions 4 Naming v.01Document52 pagesGC1 Lesson 3 Atoms Molecules and Ions 4 Naming v.01Yeri KimNo ratings yet
- LECTURE Naming CompoundsDocument63 pagesLECTURE Naming CompoundsCheri BulahanNo ratings yet
- Chemistry (Metal Compound) Notes g9Document32 pagesChemistry (Metal Compound) Notes g9Razan AbdolhaliemNo ratings yet
- Naming CompoundsDocument38 pagesNaming CompoundsYlaNo ratings yet
- Practice Problem: RevisionDocument133 pagesPractice Problem: RevisionJerry Sumok WalterNo ratings yet
- Formula Writing and Chemical Nomenclature 2Document8 pagesFormula Writing and Chemical Nomenclature 2Mark Angelo Arombo100% (1)
- Metals & Non-MetalsDocument14 pagesMetals & Non-MetalsTamoghna DeyNo ratings yet
- Grade 10 Chemical BondingDocument5 pagesGrade 10 Chemical BondingtsteadmanNo ratings yet
- Elementos QuimicosDocument1 pageElementos QuimicosKeith Ayelen Valeria Gutiérrez CaroNo ratings yet
- Metal and Non MetalsDocument7 pagesMetal and Non Metalschhabra navdeep100% (1)
- KS3 Chemistry: 9E Metals and Metal CompoundsDocument32 pagesKS3 Chemistry: 9E Metals and Metal CompoundsPedroDavid JoaquinFernandoNo ratings yet
- Writing and Naming Chemical FormulasDocument3 pagesWriting and Naming Chemical FormulasCarlo Joseph Moskito100% (1)
- 9E Reactions of Metals and Metal CompoundsDocument32 pages9E Reactions of Metals and Metal CompoundsNamoNo ratings yet
- 2 Lewis Dot Structure and Chemical BondingDocument44 pages2 Lewis Dot Structure and Chemical BondingIanna Mae Louise BaylenNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 4.5 out of 5 stars4.5/5 (2)
- A Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksFrom EverandA Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksNo ratings yet
- Jumpin at The Woodside (Warrington)Document11 pagesJumpin at The Woodside (Warrington)tylerNo ratings yet
- 2020 Calculus BC Free Response Review #1Document3 pages2020 Calculus BC Free Response Review #1tylerNo ratings yet
- POE Final Exam ReviewDocument17 pagesPOE Final Exam ReviewtylerNo ratings yet
- Activity 1.2.5 Mechanical System Efficiency - VEXDocument10 pagesActivity 1.2.5 Mechanical System Efficiency - VEXtylerNo ratings yet
- Describing Graphs NotesDocument4 pagesDescribing Graphs NotestylerNo ratings yet
- Sem 2 WK 4 Source AnalysisDocument9 pagesSem 2 WK 4 Source AnalysistylerNo ratings yet
- Lasting Effects of The Scientific Revolution and EnlightenmentDocument7 pagesLasting Effects of The Scientific Revolution and EnlightenmenttylerNo ratings yet
- Misty Medieval NightDocument6 pagesMisty Medieval NighttylerNo ratings yet
- June 2010 MS - Unit 1 Edexcel Chemistry A-LevelDocument28 pagesJune 2010 MS - Unit 1 Edexcel Chemistry A-LevelNabindra RuwaliNo ratings yet
- Pile Formula CP4 Hiley Metric PDFDocument4 pagesPile Formula CP4 Hiley Metric PDFsstibisNo ratings yet
- FOUNDATIONDocument5 pagesFOUNDATIONLYNSER CORONEL ABANZADONo ratings yet
- Crystal StructureDocument16 pagesCrystal StructurejyotiblossomsNo ratings yet
- Mobil SHC CibusDocument2 pagesMobil SHC CibusDavid LieNo ratings yet
- The Special Features of Cement Standards in China PDFDocument5 pagesThe Special Features of Cement Standards in China PDFyinglvNo ratings yet
- Tungsten Exploration in IndiaDocument24 pagesTungsten Exploration in IndiaAravind Kumaravelu100% (1)
- Defects in Plaster WorkDocument2 pagesDefects in Plaster WorksuryakantameNo ratings yet
- 2022 - Dynamic Response of RC Beam-Slab Substructures FolDocument16 pages2022 - Dynamic Response of RC Beam-Slab Substructures FolbenNo ratings yet
- A Review of Regrinding and Fine GrindingDocument27 pagesA Review of Regrinding and Fine GrindingabrahanNo ratings yet
- Aggregate Tests For Hot Mix Asphalt: State of The PracticeDocument22 pagesAggregate Tests For Hot Mix Asphalt: State of The PracticeProf. Prithvi Singh KandhalNo ratings yet
- D-Block ElementsDocument3 pagesD-Block ElementsSaksham KumarNo ratings yet
- S6 Chem Eng1Document35 pagesS6 Chem Eng1LearningNo ratings yet
- Thermal Spray CR Alternative-Limited PDFDocument28 pagesThermal Spray CR Alternative-Limited PDFhanane_220005258No ratings yet
- 10 - RC2 Lecture 5.0 - Lap SplicesDocument4 pages10 - RC2 Lecture 5.0 - Lap SplicesRichard PekitpekitNo ratings yet
- ColaDet DEF 30Document2 pagesColaDet DEF 30mndmattNo ratings yet
- BPR 12-Hsa - TDSDocument1 pageBPR 12-Hsa - TDSVaittianathan MahavapillaiNo ratings yet
- 40 KL DesignDocument10 pages40 KL DesignSung Woong MoonNo ratings yet
- Chapter 4 - Principles of Selected Joining and Assembling Process in Welding Meng 4211Document26 pagesChapter 4 - Principles of Selected Joining and Assembling Process in Welding Meng 4211Kenasa JambareNo ratings yet
- Environmental Chemistry PDFDocument3 pagesEnvironmental Chemistry PDFRijit ChakrabortyNo ratings yet
- As 2074-2003 Cast SteelsDocument8 pagesAs 2074-2003 Cast SteelsSAI Global - APACNo ratings yet
- Micro Project CPPDocument11 pagesMicro Project CPPNitesh SonaleNo ratings yet
- ASTM A234-2011aDocument8 pagesASTM A234-2011aSam WeberNo ratings yet
- Boiler Tube LeakageDocument9 pagesBoiler Tube LeakageSoham MallickNo ratings yet
- Deign Project Review 1 (120me0012)Document15 pagesDeign Project Review 1 (120me0012)VfhhdfvhhNo ratings yet
- Arcvs Technology Rev 3Document13 pagesArcvs Technology Rev 3Vinod Kumar TurkiNo ratings yet
- Castrol Seamax Super Plus Is A SAE 15W-40 Using Castrol Seamax Super PlusDocument2 pagesCastrol Seamax Super Plus Is A SAE 15W-40 Using Castrol Seamax Super PlusKaly7No ratings yet
- Q1 Week 10 Additional Activities 2020 2021 PDFDocument11 pagesQ1 Week 10 Additional Activities 2020 2021 PDFMarnelley Bautro de AldayNo ratings yet
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Uploaded by
tylerOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Combinations: Metal+Nonmetal Give+Take Electrons 2 Non Metals Nonmetal + Polyatomic Ion Electrons Are Shared
Uploaded by
tylerCopyright:
Available Formats
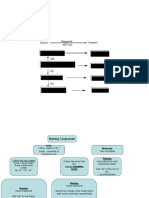
Combinations
Covalent
Ionic
2 non metals
Metal+Nonmetal
Nonmetal + polyatomic ion
Give+Take=Electrons
Electrons are shared
Polyatomic
Binary Many elements
1 metal + 1 non metal 1 metal + many nonmetals
(as a group)
Roman Numeral
(Transition) metals mostly
Simple
Simple
Metal + Nonmetal end -ied Metal+Polyatomic Ion
Roman Numeral
Metals with 1 charge option Metal + (Charge Of Metal) + nonmetal + - . Metal + charge+ nonmetal
Groups 1 and 2 end in -ide Donʼt change ending
Metals have more than 1 charge option
Often end with ate, ite, and ide
Ca(NO3)2 AuNO3
Li2 S Cu2S Calcium nitrate Gold(I)nitrate
Lithium sulfide Copper(I)sulfide
You might also like
- WORKSHEET: Chemical Bonding - Ionic & Covalent! Ionic Bond Covalent BondDocument3 pagesWORKSHEET: Chemical Bonding - Ionic & Covalent! Ionic Bond Covalent BondHaven jethro UrbanoNo ratings yet
- Ionic Bond LabDocument6 pagesIonic Bond LabHuda Wahab0% (1)
- Nmat Reviewer Gen and Ana ChemDocument22 pagesNmat Reviewer Gen and Ana Chemforfuture reviewersNo ratings yet
- HyaFilia Brochure (E) 0804-1Document12 pagesHyaFilia Brochure (E) 0804-1sha100% (1)
- .Ws Ionic Bonding Activity KeyDocument4 pages.Ws Ionic Bonding Activity KeyrajaijahNo ratings yet
- 2020 Unit 5 Notes - TEACHER (Nomenclature)Document11 pages2020 Unit 5 Notes - TEACHER (Nomenclature)Adi ChhNo ratings yet
- Chemistry - Writing Formula and Chemical EquationsDocument18 pagesChemistry - Writing Formula and Chemical EquationsexperiorNo ratings yet
- Naming Flowchart ChemistryDocument1 pageNaming Flowchart ChemistryamyNo ratings yet
- Chapter 2 Stoichiometry Edu NitpdfDocument42 pagesChapter 2 Stoichiometry Edu Nitpdfapi-386303659No ratings yet
- The Periodic TableDocument5 pagesThe Periodic TablePunyathorn KansiriNo ratings yet
- Chemical Formulas-General ChemistryDocument19 pagesChemical Formulas-General Chemistry7assan1300No ratings yet
- ROAD TO MY LEWIS ActivityDocument1 pageROAD TO MY LEWIS ActivityCristy SevillaNo ratings yet
- Types of CompoundsDocument15 pagesTypes of CompoundsJonard PedrosaNo ratings yet
- Nomenclature FlowchartDocument2 pagesNomenclature FlowchartaresamritNo ratings yet
- Formulas and Nomenclature of Ionic and Covalent Compounds: ContentsDocument13 pagesFormulas and Nomenclature of Ionic and Covalent Compounds: Contentsawesome avedNo ratings yet
- Reviewer in ChemistryDocument11 pagesReviewer in Chemistryxian tanNo ratings yet
- Naming Chemical CompoundsDocument44 pagesNaming Chemical Compoundss140917No ratings yet
- Chemistry: Writing Ionic Formulas For CompoundsDocument5 pagesChemistry: Writing Ionic Formulas For CompoundsTiffany GallinaNo ratings yet
- Naming Compounds FlowchartDocument1 pageNaming Compounds Flowchartapi-310503032No ratings yet
- Chemical Formulas KeynoteDocument34 pagesChemical Formulas Keynotewperry42No ratings yet
- Penamaan SenyawaDocument12 pagesPenamaan SenyawaJason Enduro BayuNo ratings yet
- VER2Formulas and Nomenclature of Ionic and Covalent Compounds PDFDocument12 pagesVER2Formulas and Nomenclature of Ionic and Covalent Compounds PDFjamesNo ratings yet
- 02 Polymeric Materials Molecular Lecture 2Document30 pages02 Polymeric Materials Molecular Lecture 2Katrina BucudNo ratings yet
- Ions and MoleculesDocument4 pagesIons and MoleculesITZDUSTY gamerNo ratings yet
- Chemistry TablesDocument3 pagesChemistry Tableswvcs2gz9bbNo ratings yet
- Chapter 1 - 2021-Matter and Nomenclature (Student)Document50 pagesChapter 1 - 2021-Matter and Nomenclature (Student)Micho santuna Putra putraNo ratings yet
- Chem Handout NamingDocument3 pagesChem Handout NamingAlexander DolinNo ratings yet
- Inorganic NomenclatureDocument65 pagesInorganic NomenclatureCalm your MindNo ratings yet
- Malaysha Brunner - Unit 5E Procedures For Naming Ionic CompoundsDocument5 pagesMalaysha Brunner - Unit 5E Procedures For Naming Ionic CompoundsMalaysha BrunnerNo ratings yet
- Ionic BondsDocument3 pagesIonic BondsrajaijahNo ratings yet
- 4 Chemical Formula Naming PDFDocument30 pages4 Chemical Formula Naming PDFDenise SeseNo ratings yet
- 08-Metals and Non-Metals Theory PDFDocument51 pages08-Metals and Non-Metals Theory PDFvikash singh rajpurohitNo ratings yet
- Learning GuideDocument3 pagesLearning GuideLadasha AbuevaNo ratings yet
- (CHEM) Chapter 8 - Periodic TableDocument14 pages(CHEM) Chapter 8 - Periodic TableVijay Kumar NatteyNo ratings yet
- Module 16Document16 pagesModule 16Yeng LopezNo ratings yet
- Chemis - Writing Chemical EquationDocument11 pagesChemis - Writing Chemical EquationZahra PriyonoNo ratings yet
- Naming and Formula Writing of Ionic Compounds: Ms. Ma. Norma Datu Renomeron, MAED Chemistry TeacherDocument83 pagesNaming and Formula Writing of Ionic Compounds: Ms. Ma. Norma Datu Renomeron, MAED Chemistry TeacherDave SallaoNo ratings yet
- Corrosion & Its Control & Its ControlDocument28 pagesCorrosion & Its Control & Its ControlHiren KumarNo ratings yet
- Cpe639 Lecture 4Document73 pagesCpe639 Lecture 4Aisyah Addia AzizanNo ratings yet
- Plating Tutorial 2009Document95 pagesPlating Tutorial 2009gregolgratis100% (2)
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Calculations Involving Balanced Chemical EquationsDocument107 pagesCalculations Involving Balanced Chemical Equationsmain.20002245No ratings yet
- Naming Compounds ChemistryDocument58 pagesNaming Compounds ChemistryRizalyn Padua ReyNo ratings yet
- Common IonsDocument4 pagesCommon Ionssina.aral28No ratings yet
- GC1 Lesson 3 Atoms Molecules and Ions 4 Naming v.01Document52 pagesGC1 Lesson 3 Atoms Molecules and Ions 4 Naming v.01Yeri KimNo ratings yet
- LECTURE Naming CompoundsDocument63 pagesLECTURE Naming CompoundsCheri BulahanNo ratings yet
- Chemistry (Metal Compound) Notes g9Document32 pagesChemistry (Metal Compound) Notes g9Razan AbdolhaliemNo ratings yet
- Naming CompoundsDocument38 pagesNaming CompoundsYlaNo ratings yet
- Practice Problem: RevisionDocument133 pagesPractice Problem: RevisionJerry Sumok WalterNo ratings yet
- Formula Writing and Chemical Nomenclature 2Document8 pagesFormula Writing and Chemical Nomenclature 2Mark Angelo Arombo100% (1)
- Metals & Non-MetalsDocument14 pagesMetals & Non-MetalsTamoghna DeyNo ratings yet
- Grade 10 Chemical BondingDocument5 pagesGrade 10 Chemical BondingtsteadmanNo ratings yet
- Elementos QuimicosDocument1 pageElementos QuimicosKeith Ayelen Valeria Gutiérrez CaroNo ratings yet
- Metal and Non MetalsDocument7 pagesMetal and Non Metalschhabra navdeep100% (1)
- KS3 Chemistry: 9E Metals and Metal CompoundsDocument32 pagesKS3 Chemistry: 9E Metals and Metal CompoundsPedroDavid JoaquinFernandoNo ratings yet
- Writing and Naming Chemical FormulasDocument3 pagesWriting and Naming Chemical FormulasCarlo Joseph Moskito100% (1)
- 9E Reactions of Metals and Metal CompoundsDocument32 pages9E Reactions of Metals and Metal CompoundsNamoNo ratings yet
- 2 Lewis Dot Structure and Chemical BondingDocument44 pages2 Lewis Dot Structure and Chemical BondingIanna Mae Louise BaylenNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 4.5 out of 5 stars4.5/5 (2)
- A Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksFrom EverandA Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksNo ratings yet
- Jumpin at The Woodside (Warrington)Document11 pagesJumpin at The Woodside (Warrington)tylerNo ratings yet
- 2020 Calculus BC Free Response Review #1Document3 pages2020 Calculus BC Free Response Review #1tylerNo ratings yet
- POE Final Exam ReviewDocument17 pagesPOE Final Exam ReviewtylerNo ratings yet
- Activity 1.2.5 Mechanical System Efficiency - VEXDocument10 pagesActivity 1.2.5 Mechanical System Efficiency - VEXtylerNo ratings yet
- Describing Graphs NotesDocument4 pagesDescribing Graphs NotestylerNo ratings yet
- Sem 2 WK 4 Source AnalysisDocument9 pagesSem 2 WK 4 Source AnalysistylerNo ratings yet
- Lasting Effects of The Scientific Revolution and EnlightenmentDocument7 pagesLasting Effects of The Scientific Revolution and EnlightenmenttylerNo ratings yet
- Misty Medieval NightDocument6 pagesMisty Medieval NighttylerNo ratings yet
- June 2010 MS - Unit 1 Edexcel Chemistry A-LevelDocument28 pagesJune 2010 MS - Unit 1 Edexcel Chemistry A-LevelNabindra RuwaliNo ratings yet
- Pile Formula CP4 Hiley Metric PDFDocument4 pagesPile Formula CP4 Hiley Metric PDFsstibisNo ratings yet
- FOUNDATIONDocument5 pagesFOUNDATIONLYNSER CORONEL ABANZADONo ratings yet
- Crystal StructureDocument16 pagesCrystal StructurejyotiblossomsNo ratings yet
- Mobil SHC CibusDocument2 pagesMobil SHC CibusDavid LieNo ratings yet
- The Special Features of Cement Standards in China PDFDocument5 pagesThe Special Features of Cement Standards in China PDFyinglvNo ratings yet
- Tungsten Exploration in IndiaDocument24 pagesTungsten Exploration in IndiaAravind Kumaravelu100% (1)
- Defects in Plaster WorkDocument2 pagesDefects in Plaster WorksuryakantameNo ratings yet
- 2022 - Dynamic Response of RC Beam-Slab Substructures FolDocument16 pages2022 - Dynamic Response of RC Beam-Slab Substructures FolbenNo ratings yet
- A Review of Regrinding and Fine GrindingDocument27 pagesA Review of Regrinding and Fine GrindingabrahanNo ratings yet
- Aggregate Tests For Hot Mix Asphalt: State of The PracticeDocument22 pagesAggregate Tests For Hot Mix Asphalt: State of The PracticeProf. Prithvi Singh KandhalNo ratings yet
- D-Block ElementsDocument3 pagesD-Block ElementsSaksham KumarNo ratings yet
- S6 Chem Eng1Document35 pagesS6 Chem Eng1LearningNo ratings yet
- Thermal Spray CR Alternative-Limited PDFDocument28 pagesThermal Spray CR Alternative-Limited PDFhanane_220005258No ratings yet
- 10 - RC2 Lecture 5.0 - Lap SplicesDocument4 pages10 - RC2 Lecture 5.0 - Lap SplicesRichard PekitpekitNo ratings yet
- ColaDet DEF 30Document2 pagesColaDet DEF 30mndmattNo ratings yet
- BPR 12-Hsa - TDSDocument1 pageBPR 12-Hsa - TDSVaittianathan MahavapillaiNo ratings yet
- 40 KL DesignDocument10 pages40 KL DesignSung Woong MoonNo ratings yet
- Chapter 4 - Principles of Selected Joining and Assembling Process in Welding Meng 4211Document26 pagesChapter 4 - Principles of Selected Joining and Assembling Process in Welding Meng 4211Kenasa JambareNo ratings yet
- Environmental Chemistry PDFDocument3 pagesEnvironmental Chemistry PDFRijit ChakrabortyNo ratings yet
- As 2074-2003 Cast SteelsDocument8 pagesAs 2074-2003 Cast SteelsSAI Global - APACNo ratings yet
- Micro Project CPPDocument11 pagesMicro Project CPPNitesh SonaleNo ratings yet
- ASTM A234-2011aDocument8 pagesASTM A234-2011aSam WeberNo ratings yet
- Boiler Tube LeakageDocument9 pagesBoiler Tube LeakageSoham MallickNo ratings yet
- Deign Project Review 1 (120me0012)Document15 pagesDeign Project Review 1 (120me0012)VfhhdfvhhNo ratings yet
- Arcvs Technology Rev 3Document13 pagesArcvs Technology Rev 3Vinod Kumar TurkiNo ratings yet
- Castrol Seamax Super Plus Is A SAE 15W-40 Using Castrol Seamax Super PlusDocument2 pagesCastrol Seamax Super Plus Is A SAE 15W-40 Using Castrol Seamax Super PlusKaly7No ratings yet
- Q1 Week 10 Additional Activities 2020 2021 PDFDocument11 pagesQ1 Week 10 Additional Activities 2020 2021 PDFMarnelley Bautro de AldayNo ratings yet