Professional Documents
Culture Documents
Halaman 1,2
Halaman 1,2
Uploaded by
Firda KurniaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Halaman 1,2
Halaman 1,2
Uploaded by
Firda KurniaCopyright:
Available Formats
REVIEW ARTICLE Hulmajage et.
al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
International Journal of Innovative
Pharmaceutical Sciences and Research
www.ijipsr.com
A REVIEW ON: SOFT GELATIN CAPSULE
Hulmajage sonali B*, Chavan Sushmita S., Khurde Sonali S., Shetkar Suchita L.,
R.V.Sugave
Department of Pharmaceutics, Channabasweshwar Pharmacy College, Latur,
Maharashtra, INDIA
Abstract
The purpose of this review is to special focus on the advances of soft gelatin capsule. The
term soft gelatin capsules are commonly abbreviated to 'soft gels’. Soft gelatin capsules are
the advantages over hard gelatin capsules is to make a liquid formulation containing the drug
in a one-piece outer gelatin shell. The soft gelatin capsule is also called as “one piece”.
Capsules are available in many sizes to provide dosing flexibility. Unpleasant drug tastes and
odors can be masked by the tasteless gelatin shell. They are suitable for encapsulation of lipid
solutions, fish oil, suspensions, or paste-like formulations, making them a useful option when
formulating poorly water-soluble drugs. This will inherently lead to better absorption of the
active ingredient as compared with delivery in a tablet or as a powder. Development of soft
gelatin capsule (soft gel) dosage form is of growing interest for the oral delivery of poorly
water soluble compounds (BCS class II or class IV). This review discusses establishment and
the on-going development of the manufacturing technology for liquid fill capsules with focus
on progress and challenges of soft gelatin capsules formulation in oral administration for
improved solubility and as an absorption-enhancing technique.
Keywords: Soft gelatin, capsules, soft gels, unpleasant, fish oil.
Corresponding Author:
Hulmajage sonali
Department of Pharmaceutics,
Channabasweshwar Pharmacy College,
Latur, Maharashtra, INDIA
E-mail: shulmajage208@gmail.com
Phone: +91-7264080082
Available online: www.ijipsr.com June Issue 59
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
INTRODUCTION
Capsule is the most versatile of all dosage forms. Capsules are solid dosage forms in which
one or more medicinal and inert ingredients are enclosed in a small shell or container usually
made of gelatin. There are two types of capsules, “hard” and “soft”. The hard capsule is also
called “two pieces” as it consists of two pieces in the form of small cylinders closed at one
end, the shorter piece is called the “cap” which fits over the open end of the longer piece,
called the “body”. The soft gelatin capsule is also called as “one piece”. Capsules are available
in many sizes to provide dosing flexibility. Unpleasant drug tastes and odors can be masked
by the tasteless gelatin shell. The administration of liquid and solid drugs enclosed in hard
gelatin capsules is one of the most frequently utilized dosage forms [1].
Advantages of Capsules:
Capsules mask the taste and odor of unpleasant drugs and can be easily administered.
They are attractive in appearance
They are slippery when moist and, hence, easy to swallow with a draught of water.
As compared to tablets less adjuncts are required.
The shells are physiologically inert and easily and quickly digested in the
gastrointestinal tract.
They are economical
They are easy to handle and carry.
The shells can be opacified (with titanium dioxide) or colored, to give protection from
light.
Manufacture Liquids can be encapsulated (non water soluble).
Small to large sizes possible.
Improved drug absorption.
Easy to swallow.
Avoids dust handling problems during manufacture and better operator safety
Overcome problems with manufacture (e.g. oils, low melting point drugs) as
compressed tablet.
Dose uniformity for low-dose drugs.
Good product stability
Disadvantages of Capsules
The drugs which are hygroscopic absorb water from the capsule shell making it brittle
and hence are not suitable for filling into capsules.
Available online: www.ijipsr.com June Issue 60
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
The concentrated solutions which require previous dilution are unsuitable for capsules
because if administered as such lead to irritation of stomach.
Soft gelatin capsules are not easily prepared except on a large scale and with
specialized equipment.
They are an expensive dosage form, when compared with direct compression tablets.
There is a more intimate contact between the shell and its liquid contents than exists
with dry-filled hard gelatin capsules, which increases the possibility of interactions.
BASIC COMPONENTS OF SOFT GELATIN SHELL [2,3]
Capsule Shell Composition
The shell of a soft gelatin capsule is composed of gelatin, a plasticizer or a combination of
plasticizers and water. In addition, it may contain preservatives, colouring and opacifying
agents, flavourings and sweeteners, possibly sugars to impart chewable characteristics to the
shell, gastro-resistant substances and in special cases even active compounds.
Water: The ratio 45% w/w by weight of water to dry gelatin can vary depending from 0.71.3
(water) to 1.0 (dry gelatin) depending on the viscosity of the gelatin being used.
Plasticizer: Used to make the soft gel shell elastic &pliable. Ration used is between 0.3- 1.8
for soft to hard shell on dry basis. e.g. Glycerin and sorbitol.
Coloring Agent: Colour used in shell has to be darker than color of encapsulating material.
Color may be natural or synthetic.
Opacifiers: Usually used is titanium dioxide, may be added to produce an opaque shell, when
the fill formulation is a suspension or to prevent photo degradation of light sensitive fill
ingredients. Concentration of opacifiers may be up to 0.5%.
Chelating agents: Iron is always present in raw gelatin & should not contain iron more than
15 ppm. Additionally chelating agent may be used for preventing the reaction of iron with
materials or colours.
Gelatin: There are two main types of gelatin: Type A: produced by acid hydrolysis of animal
skins. Type B: produced by basic hydrolysis of bovine bones. A soluble biological fluid at
body temperature. It is a good film forming material. Solutions of high concentration, 40%
w/v, are mobile at 50°C.
FORMULATION FOR SOFT GELATIN CAPSULES [4,5]
It involves liquid, rather than powder technology. Materials are generally formulated to
produce the smallest possible capsule consistent with maximum stability, therapeutic
effectiveness and manufacture efficiency. The liquids are limited to that do not have an
Available online: www.ijipsr.com June Issue 61
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
adverse effect on gelatin walls. The pH of the liquid can be between 2.5 and 7.5. Emulsion
cannot be filled because water will be released that will affect the shell.
Vehicles used in soft gelatin capsules Water immiscible, volatile or more likely volatile
liquids such as vegetable oils, mineral oils, medium chain triglycerides and acetylated
glycosides. Water miscible, non-volatile liquids such as low molecular weight PEG have come
into use more recently because of their ability to mix with water readily and accelerate
dissolution of dissolved or suspended drugs. All liquids used for filling must flow by gravity
at a temperature of 35° C or less. The sealing temperature of gelatin films is 37- 40° C.
TYPES OF FILL MATRIXES [6]
1) Lipophillic liquids / oils: e.g. Soya bean oil.
2) Hydrophilic liquids: PEG400.
3) Self-emulsifying oils (oil +nonionic surfactant).
4) Micro emulsion and Nanoemulsion systems and suspension.
Fig. 1: Shows flow chart of manufacturing of soft gelatin capsules
MANUFACTURING PROCESS OF SOFT GELATIN CAPSULE
Manufacturing process of soft gelatin process divided into some steps, there are:
1. Gelatin Preparation
2. Material (Fill) Preparation
3. Encapsulation
4. Drying
5. Inspection
6. Polishing
7. Packaging
Available online: www.ijipsr.com June Issue 62
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
1. Gelatin Preparation [7]
Raw granular gelatin is mixed with glycerin and water. Glycerin acts as a plasticizer in the
gelatin compound. Other plasticizers can also be used either alone or in combination with
glycerin, such as sorbitol. Colouring agent can also be added at this stage. The proportions of
each ingredient involved in the mixture should be considered carefully because the shell
material needs to be adapted to formulation and/or environmental requirements. For instance
the gelatin recipe may need to be adjusted to account for acidity, water content of the fill
material or high humidity environmental conditions.
Fig.2: Gelatin melting tank
The mixture while a very high torque tribune mixer stirs it under vacuum. At this stage,
approximately 20% of gelatin mixture consists of water this process takes around 3 hours until
the gelatin turns into a molten liquid mass. As soon as the liquid gelatin mass is ready for
encapsulation process, it is transferred to ground heated tanks which are wheeled into the
clean room where the main encapsulation machine is. Because the only way to keep the
gelatin mixture as liquid is to keep it warm otherwise it will solidify like jelly. It is really
important to plan and schedule the gelatin production in terms of time and quantity. At least, it
requires 2 shift operation
2. Material (Fill) Preparation [5]
A homogeneous fill material plays a vital role to ensure the uniformity of each soft gel dose.
Various equipment’s should be available such as processing tanks, high-shear mixer,
homogenizer and variety of mills is used.
There are two types of fill materials
Oil mixtures or pastes. Oil mixtures are very easy to formulate. The oils are mixed, deposited
into a ground material tank and moved into the hopper of the encapsulation machine (i.e.,
Available online: www.ijipsr.com June Issue 63
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
vitamin, fish oils).Pastes are oils or polyethylene glycols added with powders. Two important
factors that affect the homogeneity and flow of the paste should be considered:
1. Particular size of powder in order to allow homogeneous mixture, powder particles must not
be thicker than 80 mesh.
2. Viscosity of the mixture. If the mixture is not thin enough, it will not flow correctly through
the machine injections.
Natural or artificial flavors, sweeteners and fragrances are commonly used to make chewable
soft gels or to mask the unpleasant taste and Odour of the fill material such as fish oils. These
can go into fill or gelatin material
3. Encapsulation [8]
Encapsulation is the manufacturing process that brings the gelatin shell and the fill material
together to form Soft gel capsules. It takes place in a closed environment called clean room
where the relative humidity is around 20%. The gelatin shell and fill material are brought
together simultaneously in the encapsulation machine. The process is basically performed as
described; a pump delivers the warm gelatin over two chilled drums which are located at both
opposite sides of the machine, through a spreader box that sits over each drum. The revolving
stainless steel drum is about 24” in diameter and exposed to 400 CFM of 57-59°F air at 20%
RH. The warm liquid gelatin flows over the drums and this transforms the liquid gelatin into
two solid ribbons of gel. The left and right ribbons pass over rollers which feed them through
two die rolls. These die rolls determine the shape and size of soft gels and cut the Soft gel
shell from the ribbons as they turn around
Fig.3: Rotary dies filling machine
Available online: www.ijipsr.com June Issue 64
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
A sensitive and high accuracy, positive displacement pump delivers the fill materials into a
heated wedge which sits between rotary dies. injects the fill material into the die cavities
between ribbons just right before the die rolls cut the ribbons and seal the two halves together.
The cool, dry air congeals the gelatin as the drum rotates so that a tacky, elastic band rolls off
of the other end. This thin band is then automatically formed into capsules; filled with
medicine, vitamins or other products sealed and dropped into a tray. If the air blowing against
the drum has too low a temperature, the gelatin will set too rapidly and humidity are too high,
or the air become brittle which can cause the manufacturing process to grind to a halt. Too
high of an air velocity will disturb the consistent thickness of the gelatin ribbon being formed.
If the air temperature velocity is too low, the gelatin will not solidify into a ribbon. Thus, the
need for constant control of the air being introduced to the drum is critical in the process.
From the capsulating machines, the soft &moist capsules are transferred to drying drums or
chambers for rapid drying. The extent of moisture to be removed during drying depends upon
the size of the capsule, the number of capsules, and the period of time over which this
moisture can be removed.
4. Drying
Drying process purpose is to decrease the moister content to create a hard and durable finished
soft gel capsules ready for packaging. After the soft gels are formed through the die rolls, they
contain around 20 percent water. This amount of water content is needed to keep the gel
flexible enough to form the capsules. Drying process requires an environment with low
relative humidity in the air but not hot air.
Fig.4: Fluid bed tumble drier for soft gel
This process divided into two stages
First stage: Performed by a tumble dryer consists of sections. This equipment tumbles the soft
gels around 30 to 40 minutes and removes approximately 25 percent of the water content in
the soft gel capsules
Second stage: Soft gel capsules are spread on stackable trays and transferred to the drying
room or tunnel where high air flow exists and they stay around 24 to48 hours or until the soft
Available online: www.ijipsr.com June Issue 65
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
gels become hard enough. This process is called natural manual drying. By using a fully
automatic Soft gel drying machine, this long drying process time can be reduced to a few
hours which enables you to save time and money typically the environment to be maintained
for effective and rapid drying corresponds to a dew point of 25° F or an absolute humidity
level of 20 grains per pound of air. The following are typical design conditions: Temperature
Humidity 78°F 15% RH 68°F 20% RH In order to achieve the controlled air requirement
listed above, refrigeration equipment alone becomes uneconomical, impractical and
cumbersome to design, operate and maintain. On the other hand desiccant type dehumidifiers
combined with refrigeration can offer a simple and economical solution to controlling both
temperature and humidity levels as low as necessary Dry-Air desiccant dehumidifiers have
been utilized in many capsulating and soft gel manufacturing applications all over the world
resulting in millions of dollars saved annually.
5. Inspection& In Process Control for Finished Product [9]
Due to air pockets in the gelatin and fill material and district production tolerances, the Soft
gel capsules may vary in size and need to be inspected visually. Any misshaped, damaged
and/or not fully filled capsules are removed manually by using an inspection table. Manual
inspection process can be preferred if you have small batch size production but if you intend
to manufacture soft gel capsules in mass quantities. Manual inspection will be time consuming
process which leads to accuracy problems. In order to reduce process time and increase the
accuracy, fully automatic soft gel sorting machine equipped with electronic sensors can be
used to sort and remove the damaged, misshaped, broken etc. gelatin capsules. Two to three
percent is an acceptable reject rate.
In-process testing
During the encapsulation process the four most important tests are:
1) The gel ribbon thickness.
2) Soft gel seal thickness at the time of encapsulation.
3) Fill matrix weight & capsule shell weight.
4) Soft gel shell moisture level and soft gel hardness at the end of the drying stage.
Finished product testing:
1) Capsule appearance.
2) Active ingredient assay &related substances assay.
3) Fill weight. Content uniformity.
4) Microbiological testing
Available online: www.ijipsr.com June Issue 66
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
6. Polishing
The final step before packaging is to clean and polish the Soft gel capsules to remove any
mineral oil or glycerin that the capsules may have on their exterior skin. Tumbling is the most
used production method to clean the Soft gel capsules among others such as washing with
solvent.
7. Packaging
There is no difference between packaging soft gels and traditional tablets or hard capsules.
Any finished Soft gel product should be stored in an environment with temperature round 20-
24 °C and relative humidity 35%.
Fig.5: Different shapes of soft gelatin capsule
FORMULATION FACTORS AFFECTING DRUG AVAILABILITY FROM
CAPSULES
The overall dissolution rate of a drug from capsules may be regarded as a Function of several
variables:
1) The dissolution rate of the shell.
2) The rate of penetration of the dissolution medium into the powder.
3) The rate at which the powder mass de aggregates.
4) The amount and nature of adjuvant such as diluents, surfactant (infused).
5) Drug particle size.
6) The composition and characteristics of the capsule shell.
ADVANCES AND CHALLENGES IN SOFTGEL CAPSULES DEVELOPMENT
Ad Soft gelatin capsules have received enormous consumer acceptance due to their numerous
advantages over other traditional dosage forms such as tablets and even hard capsules [10]. It
has become not uncommon for pharmaceutical companies to reformulate marked solid dosage
Available online: www.ijipsr.com June Issue 67
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
form in the form of soft gelatin capsules in order improve consumer acceptability owing to
dissolution in the gastrointestinal tract of drugs formulated as soft gelatin capsules [11].
Several advances have been made in the development of soft gel capsules including [12]
Ensuring of suitable physical features. The soft gelatin capsule has been developed to
have a tidy and beautiful appearance compared to tablet pills. These features have been
reported to ensure faster disintegration speed, higher bioavailability and convenient
ingestion.
The capsule can cover unpleasant odor of the medicine.
The capsule has been made into instant-effective, slow-releasing, enteric coated or
gastro coated soft gelatin capsule.
Achievement has been reported in formulation of medicines with dosage form of
higher oil concentration that cannot be easily made into tablets or pills and small
dosage principle agent that is not water-soluble and hard to be ingested in the
digestive.
Soft capsules technology have facilitated light sensitive and unstable drugs to be filled
into opaque light proof opageopa capsule to improve the stability and prevent the drug
from been affected by moisture, oxygen and light in the air.
CHALLENGES IN DEVELOPMENT OF SOFT GELATIN CAPSULE
Although the formulation of drugs into soft gel capsules has been reported to solve many
problem associated with tableting including poor compaction, lack of content or weight
uniformity, and other powder flow or mixing problems [13], several problems affecting the
development of soft gel capsules have been revealed.
Poor differentiation and /or mismatching of gel material added to cross-linking with the drug.
The stability of an API in a liquid dosage form, as opposed to a solid dosage form is, in itself,
a challenge, as is matching the formulation with the characteristics of the capsule shell and the
dosing system of the capsule filling machine. In cases where gelatin as a capsule material is
not suitable, alternative polymers for capsule production may be exploited [14].
Another phenomenon seen in capsules, especially soft gels is extensive cross-linking to the
gelatin, which could occur during storage and result in the formation during dissolution testing
of swollen, rubbery water-insoluble membranes known as pellicles that have been reported to
act as barrier to drug release [15].
The major challenge in the development of the soft gel dosage form is that the system is very
dynamic in terms of;
Available online: www.ijipsr.com June Issue 68
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
1) The physical migration of components between the shell and the fill and the shell and the
external environment,
2) The occurrence of physical and chemical interactions within and between the shell and fill
components [16].
EVALUATION OF COMMERCIAL CAPSULES [17]
1) Content uniformity.
2) Weight Uniformity.
3) Disintegration and
4) Dissolution
Content uniformity
1)30 capsules are selected and10 of these are assayed individually.
2) At least 9 of these contain 85–115% of drug and none contain below 75–125% of drug.
3)If 1to 3of them fall outside of85–115% limits, the remaining20 capsules are individually
assayed and the requirements are met if no few than 27Contain 85– 115% of drug and none
contain less than 75–125% of drug
Weight Uniformity
This test applies to all types of capsules and it is to be done on 20capsules.
Method
1) Weigh an intact capsule.
2) Open the capsule without losing any part of the shell and remove the contents as completely
as possible.
3) Weigh the shell.
4) The weight of the contents is the difference between the weighing.
5) Repeat the procedure with a further 19 capsules Selected at random.
6) Determine the average weight.
Disintegration
The disintegration test determines whether tablets or capsules disintegrate with in a prescribed
time when placed in a liquid medium under the prescribed experimental conditions.
Method
According to the B.P. and applies to hard and soft capsules.
1) Introduce one capsule into each tube and suspend the apparatus in a beaker containing 600
ml water at 37°C. If hard capsules float on the surface of the water, the discs may be added.
2) Operate the apparatus for 30 minutes; remove the assembly from the liquid.
3) The capsules pass the test if,
Available online: www.ijipsr.com June Issue 69
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
No residue remains on the screen of the apparatus or, If a residue remains, it consists of
fragments of shell If a soft mass with no palpable core.
If the disc is used, any residue remaining on its lower surface should only consist of fragments
of shell.
Dissolution
1) The dissolution test is carried out using the dissolution apparatus official in both the U.S.P.
and N.F.
2) The capsule is placed in a basket formed from 40-meshstainless steel fabric.
3) A stirrer shaft is attached to the basket, and the basket is immersed in the dissolution
medium and caused to rotate at a specified speed.
4) The dissolution medium is held in a covered1000 ml glass vessel and maintained
at37°C±0.5°C by means of a suitable constant- temperature water bath.
5) The stirrer speed and type of dissolution medium are specified in individual monograph.
APPLICATIONS
1) As an oral dosage form of ethical or proprietary products for human or veterinary use.
2) As a specialty package in the tube form, for human and veterinary single dose application
of topical, ophthalmic, and optic preparations, and rectal ointments
CONCLUSION
Interesting advances have recently been made in the area of developing liquid and semi-solid
formulation in a soft gelatin capsule to address particular bio performance issues, namely an
increase of bioavailability and decrease of plasma variability by improving solubility and
absorption enhancing techniques. Although the soft gel capsules have many advantages, they
also face stability problem, mainly for the soft capsules stored for longer than six months.
After this time, their soluble products decreased and the remnants cannot be re-dissolved
and/or reabsorbed into the gastrointestinal tract.
REFERENCES
1. Lachman & Lieberman. The theory and practice of Pharmacy. New Delhi: CBS
publisher; 817- 823.
2. Jones RT. Gelatin: manufacture and Physico-chemical properties. In: Podczeck F,
Jones B E (Eds) Pharmaceutical capsules. London: Pharmaceutical Press; 2004; 23-60
3. Hom FS, Veresh SA, Ebert WR. Soft gelatin capsules II: Oxygen permeability study of
capsule shells. Journal of pharmaceutical sciences 1975; 64(5): 851-7.
4. Brox W. Pharmaceutical compositions. EP. 1983; 0121321.
Available online: www.ijipsr.com June Issue 70
REVIEW ARTICLE Hulmajage et.al / IJIPSR / 6 (06), 2018, 59-71
Department of Pharmaceutics ISSN (online) 2347-2154
DOI: 10.21276/IJIPSR.2018.06.06.310
5. Brox W, Zande H, Meinzer A. Soft gelatin capsule manufacture. EP 1993; 0 649- 651.
6. Alton’s Pharmaceutics, The design and manufacture of medicines. 3rd ed. Churchill
Livingstone; 2007; 538.
7. Aulton’s Pharmaceutics. The design and manufacture of medicines. 3rd ed. Churchill
Livingstone; 2007; 517.
8. Brox, W. Soft gelatin capsules and methods for their production. US Patent, 1988; 4
744 988.
9. Ferdinand JC. Formulation solutions Soft gels. Pharmaceutical Manufacturing and
Packaging Source Spring Issue, 2000; 69-73
10. Colea ET, Cadé D, and Benameur H. Challenges and opportunities in the
encapsulation of liquid and semi-solid formulations into capsules for oral
administration, Advanced Drug Delivery Reviews 2008, 60: 747
11. Frutos G, Prior-Camarillas A, París R and Quijada-Garrido I. A novel controlled drug
delivery system based on pH-responsive hydrogels included in soft gelatin capsules.
Acta Biomaterial 2010, 6 (12): 4650-4656
12. Mei X, Etzler FM and Wang Z. Use of texture analysis to study hydrophilic solvent
effects on the mechanical properties of hard gelatin capsules. International Journal of
Pharmaceutics. 2006, 324: 128–135
13. Colea ET, Cadé D, and Benameur H. Challenges and opportunities in the
encapsulation of liquid and semi-solid formulations into capsules for oral
administration, Advanced Drug Delivery Reviews 2008, 60: 747–756
14. Rossi RC, Dias CL, Donato EM, Martins LA, Bergold AM and Froehlich PE.
Development and validation of dissolution test for ritonavir soft gelatin capsules based
on in vivo data. International Journal of Pharmaceutics 2007, 338: 119–124.
15. Donato EM, Martins LA, Froehlich PE and Bergold AM. Development and validation
of dissolution test for lopinavir, a poorly water-soluble drug, in soft gel capsules, based
on in vivo data, Journal of Pharmaceutical and Biomedical Analysis 2008, 47: 547–
552.
16. Martins GZ, Souza CRF, Shankar TJ and Oliveira WP. Effect of process variables on
fluid dynamics and adhesion efficiency during spouted bed coating of hard gelatin
capsules. Chemical Engineering and Processing 2008, 47: 2238–2246.
17. Ansel’s. Pharmaceutical Dosage Forms and Drug Delivery Systems.8th ed. New Delhi:
B.I.Publications; 2006; 218-219.
Available online: www.ijipsr.com June Issue 71
You might also like
- IRJIPS MY 14 06DeepakGargDocument12 pagesIRJIPS MY 14 06DeepakGargjeyapragash RamadassNo ratings yet
- Soft Gel FormulationDocument12 pagesSoft Gel FormulationIndu nutraNo ratings yet
- Soft Gelatin Capsules-Present and FutureDocument10 pagesSoft Gelatin Capsules-Present and Futurelouish9175841No ratings yet
- CAPSULESDocument35 pagesCAPSULESde_rigorry67% (3)
- Evaluation Parameters of Hard and Soft Gelatin Capsule and Their Manufacturing ProcessDocument17 pagesEvaluation Parameters of Hard and Soft Gelatin Capsule and Their Manufacturing ProcessRahul VermaNo ratings yet
- Types, Manufacture, Formulation of Capsules 1Document26 pagesTypes, Manufacture, Formulation of Capsules 1chill streamNo ratings yet
- Capsules and It's Technology: An Overview: ArticleDocument8 pagesCapsules and It's Technology: An Overview: ArticleR TicoaluNo ratings yet
- Excipients (Pharma Homework)Document6 pagesExcipients (Pharma Homework)Ian KoNo ratings yet
- Soft Gelatin Capsules (Softgels)Document8 pagesSoft Gelatin Capsules (Softgels)Abhishek RajNo ratings yet
- V. Deva Prasad V. Deva Prasad V. Deva PrasadDocument15 pagesV. Deva Prasad V. Deva Prasad V. Deva PrasadAhmad RazaNo ratings yet
- Application of Pharmaceutical GelatinDocument4 pagesApplication of Pharmaceutical GelatinShakrie AbdullahNo ratings yet
- Capsules .Document40 pagesCapsules .Phar MacyNo ratings yet
- Capsules and It's Technology PDFDocument7 pagesCapsules and It's Technology PDFlouish9175841No ratings yet
- CapsulesDocument84 pagesCapsuleskarthikeyan V100% (1)
- Patented Technology in Soft Gelatin Capsule A Review PDFDocument16 pagesPatented Technology in Soft Gelatin Capsule A Review PDFlouish9175841No ratings yet
- Industrial Pharmaceutical Tecnology: (Essentials)Document35 pagesIndustrial Pharmaceutical Tecnology: (Essentials)davidNo ratings yet
- A Review of Progress and Challenges in Soft Gelatin Capsules Formulations For Oral AdministrationDocument6 pagesA Review of Progress and Challenges in Soft Gelatin Capsules Formulations For Oral Administrationlouish9175841No ratings yet
- Capsules: Industrial Pharmacy H. TizgamDocument8 pagesCapsules: Industrial Pharmacy H. TizgamMustafa Salah MahdiNo ratings yet
- Dosage - Chapter 7Document6 pagesDosage - Chapter 7kaukau4everNo ratings yet
- A Review - Capsule Shell Material From Gelatin To Non Animal OriginDocument30 pagesA Review - Capsule Shell Material From Gelatin To Non Animal OriginAyten Güllüce100% (1)
- Patented Technology in Soft Gelatin Capsule A ReviewDocument16 pagesPatented Technology in Soft Gelatin Capsule A ReviewFatimahAhmat100% (1)
- Capsules ReportDocument31 pagesCapsules ReportkdvillanuevaNo ratings yet
- Chapter 7 CapsulesDocument87 pagesChapter 7 CapsulesTeresa Saylo93% (27)
- Formulation and In-Vitro Characterization of Calcitriol Soft Gelatin CapsuleDocument23 pagesFormulation and In-Vitro Characterization of Calcitriol Soft Gelatin CapsuleArief Adi NugrohoNo ratings yet
- 834 Ijar-10870 PDFDocument6 pages834 Ijar-10870 PDFBhavin VoraNo ratings yet
- Soft Gelatin Capsules (SoftgelsDocument42 pagesSoft Gelatin Capsules (Softgelsprenatooliveira91% (11)
- Soft Get and Hard Gel CapsulesDocument8 pagesSoft Get and Hard Gel CapsulesMahmuda Akter Marzia 2014151649No ratings yet
- Solid Dosage Form Part 2Document44 pagesSolid Dosage Form Part 2Claire Marie AlvaranNo ratings yet
- Formulation and Physical Properties of Soft Capsules: Gabriele ReichDocument12 pagesFormulation and Physical Properties of Soft Capsules: Gabriele ReichYuppie RajNo ratings yet
- (Vinesia, 2010: Importance of CapsuleDocument8 pages(Vinesia, 2010: Importance of CapsuleFarzana ShantaNo ratings yet
- Stability Study of Soft Gelatin CapsuleDocument3 pagesStability Study of Soft Gelatin CapsuleAlyanna Hope TondoNo ratings yet
- Capsule Mod 2Document9 pagesCapsule Mod 2Manasvi MehtaNo ratings yet
- Capsules Part-3 MaterialDocument36 pagesCapsules Part-3 Materialsimbi beliseNo ratings yet
- Gullapalli, 2009, Soft Gelatin CapsulesDocument42 pagesGullapalli, 2009, Soft Gelatin CapsulesAnggit Saputra Dwipramana100% (1)
- CriticDocument2 pagesCriticJohnAlsandairAgcaoiliNo ratings yet
- Gelatin and Non Gelatin Soft Gel Capsules A ReviewDocument11 pagesGelatin and Non Gelatin Soft Gel Capsules A Reviewgeneracionlcr963700No ratings yet
- Soft Cap-2Document36 pagesSoft Cap-2DrSambathkumar RamanathanNo ratings yet
- VINDocument22 pagesVINsky998493No ratings yet
- Pharmaceutical Capsule Solid Dosage FormDocument16 pagesPharmaceutical Capsule Solid Dosage FormRasla MolNo ratings yet
- Capsu LES: Pharma Ceutics 2Document14 pagesCapsu LES: Pharma Ceutics 2Mirumbi Kefa MomanyiNo ratings yet
- Recent Innovation in CapsulesDocument23 pagesRecent Innovation in Capsuleslucky5papa08No ratings yet
- Formulation and Development of Sustained Release Matrix Tablets of LornoxicamDocument6 pagesFormulation and Development of Sustained Release Matrix Tablets of LornoxicamRYP KBHIOPNo ratings yet
- ProjectDocument27 pagesProjectAbhishek YadavNo ratings yet
- Capsulas BlandasDocument11 pagesCapsulas BlandasClaudia DanielaNo ratings yet
- CAPSULES EditedDocument14 pagesCAPSULES Editedmelvin karanjaNo ratings yet
- 5 Vol. 5 Issue 6 June 2014 IJPSR RE 1270 Paper 5Document9 pages5 Vol. 5 Issue 6 June 2014 IJPSR RE 1270 Paper 5Lilis TuslinahNo ratings yet
- ArticleDocument12 pagesArticleJiang ZhangNo ratings yet
- 5 - CapsulesDocument20 pages5 - CapsulesAl-Homam SalahNo ratings yet
- CapsuleDocument71 pagesCapsuleMuhammad TalhaNo ratings yet
- Cole 1999 Liquid Filled and Sealed Hard Gelatin CapsulesDocument12 pagesCole 1999 Liquid Filled and Sealed Hard Gelatin CapsulesZirrus GlassNo ratings yet
- 704 712Document9 pages704 712Jorge Humberto HerreraNo ratings yet
- Formulation and Physical Properties of Soft Capsules (Gabriele Reich)Document13 pagesFormulation and Physical Properties of Soft Capsules (Gabriele Reich)coooleNo ratings yet
- Enzymes in The Dissolution Testing of Gelatin CapsulesDocument7 pagesEnzymes in The Dissolution Testing of Gelatin CapsuleselvaNo ratings yet
- Introduction and Formulation: Preparation of SuppositoryDocument52 pagesIntroduction and Formulation: Preparation of SuppositoryImam BisriNo ratings yet
- Fast Dissolving Tablet - A ReviewDocument7 pagesFast Dissolving Tablet - A ReviewPharmacognosy JournalNo ratings yet
- CapsuleskncopDocument181 pagesCapsuleskncopboozokaranNo ratings yet
- Oral Disintegrating Tablets A Future CompactionDocument10 pagesOral Disintegrating Tablets A Future CompactionArifin I. OputuNo ratings yet
- Handbook of Polymers for Pharmaceutical Technologies, Structure and ChemistryFrom EverandHandbook of Polymers for Pharmaceutical Technologies, Structure and ChemistryNo ratings yet
- Fatigue Assessment of Weld Joints Using ANSYS, Verity & FE-SafeDocument32 pagesFatigue Assessment of Weld Joints Using ANSYS, Verity & FE-SafeDeepak Agarwal100% (1)
- ACI Method of Concrete Mix Design - Procedure and CalculationsDocument8 pagesACI Method of Concrete Mix Design - Procedure and Calculationsvenkateswara rao pothinaNo ratings yet
- 4th Quarter - ScienceDocument26 pages4th Quarter - ScienceGian BaliloNo ratings yet
- Miklos N Szilagyi's Curriculum VitaeDocument8 pagesMiklos N Szilagyi's Curriculum VitaeMiklos N. SzilagyiNo ratings yet
- An Experimental Investigation On Stabilized Mud MortarDocument9 pagesAn Experimental Investigation On Stabilized Mud MortarasyreenhaikalNo ratings yet
- Production of Aluminum-Silicon Carbide Composites Using Powder Metallurgy at Sintering Temperatures Above The Aluminum Melting PointDocument13 pagesProduction of Aluminum-Silicon Carbide Composites Using Powder Metallurgy at Sintering Temperatures Above The Aluminum Melting PointMustafa Levent SipahiNo ratings yet
- Self Organising System by AshbyDocument25 pagesSelf Organising System by AshbyczhenlinNo ratings yet
- High-Shear Viscosity Using A Cone/Plate Viscometer: Standard Test Method ForDocument3 pagesHigh-Shear Viscosity Using A Cone/Plate Viscometer: Standard Test Method ForgizemNo ratings yet
- S-Block Elements: Earth Metals. These Are So Called Because Their Oxides and Hydroxides Are Alkaline in NatureDocument8 pagesS-Block Elements: Earth Metals. These Are So Called Because Their Oxides and Hydroxides Are Alkaline in NatureAgamGoelNo ratings yet
- Bernoulli Principle K-4Document38 pagesBernoulli Principle K-4Shirat MohsinNo ratings yet
- Insructions Trad Part 2Document25 pagesInsructions Trad Part 2codruta_holtzNo ratings yet
- Graftech Engineering ManualDocument117 pagesGraftech Engineering ManualroscillaNo ratings yet
- CHEM F111 General Chemistry: PilaniDocument57 pagesCHEM F111 General Chemistry: PilaniSunil NahataNo ratings yet
- Li-2018 - Flatness Control Capability of Cold Rolling MillDocument13 pagesLi-2018 - Flatness Control Capability of Cold Rolling Milldean chenNo ratings yet
- Catappa) and Its Fruits Are Scattered EverywhereDocument18 pagesCatappa) and Its Fruits Are Scattered EverywhereRichelle ValienteNo ratings yet
- ch13 AnsDocument10 pagesch13 AnsTân Dương100% (1)
- Motovario MaintenanceDocument72 pagesMotovario Maintenancearachman297988No ratings yet
- Analysis of Rotary Intersection at Vadodara (India)Document5 pagesAnalysis of Rotary Intersection at Vadodara (India)IJSTE100% (1)
- RKP4, RKP8 & RKP16 Keypads Installation ManualDocument4 pagesRKP4, RKP8 & RKP16 Keypads Installation ManualgysiedebruynNo ratings yet
- Astm C1698 09Document4 pagesAstm C1698 09Shivam SinghNo ratings yet
- WST ET 45 Micro 161109 PDFDocument8 pagesWST ET 45 Micro 161109 PDFBehroozNo ratings yet
- C 71826 Ab 108691 Eeabc 7Document26 pagesC 71826 Ab 108691 Eeabc 7api-262225822No ratings yet
- Formula Derivation: Stefan'S LawDocument7 pagesFormula Derivation: Stefan'S LawRitesh BhoiteNo ratings yet
- Wave MotionDocument13 pagesWave MotionGDGGFGFNo ratings yet
- Unit-V (Topic - 6 To 10)Document71 pagesUnit-V (Topic - 6 To 10)vsresika20No ratings yet
- SDM 102 eDocument24 pagesSDM 102 eseaqu3stNo ratings yet
- Electrical Grounding Architecture For Unmanned Spacecraft: Nasa Technical HandbookDocument29 pagesElectrical Grounding Architecture For Unmanned Spacecraft: Nasa Technical HandbookSarah TanNo ratings yet
- 68 10.1007@s00603-019-01774-zDocument20 pages68 10.1007@s00603-019-01774-zeulogiotangNo ratings yet
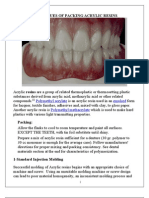
- Techniques of Packing Acrylic Resins (My Seminar"Muhammad Magdi Bishr" @AmCoFam)Document12 pagesTechniques of Packing Acrylic Resins (My Seminar"Muhammad Magdi Bishr" @AmCoFam)AmericanCornerFamilyNo ratings yet
- Tiruvannamalai District Graph: 10 STD Mathematics-Algebra Ex 3.16Document14 pagesTiruvannamalai District Graph: 10 STD Mathematics-Algebra Ex 3.16PoomalaiNo ratings yet