Professional Documents
Culture Documents
Aldol Condensation Reaction PDF
Aldol Condensation Reaction PDF
Uploaded by
aizatOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aldol Condensation Reaction PDF
Aldol Condensation Reaction PDF
Uploaded by
aizatCopyright:
Available Formats
lOMoARcPSD|3377713
Aldol condensation reaction
Chemistry (Trinity College Dublin University of Dublin)
StuDocu is not sponsored or endorsed by any college or university
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
lOMoARcPSD|3377713
Experiment 1
Aldol Condensation reactions
Introduction
(a) Objectives
To synthesize Dibenzalacetone.
To synthesize Benzalacetophenone.
To measure the melting point of the sample.
To understand the mechanism of an Aldol Reaction.
(b) Theory
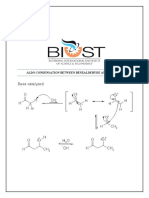
An Aldol condensation reaction is one in which an enol or an enolate ion reacts
with a Carbonyl compound to form β-hydroxyaldehyde or a β-hydroxyketone,
hence a dehydration gives a conjugate enone. In both parts of this practical
experiment Benzaldehyde reacts with a ketone in the presence of a base to
produce β-unsaturated ketones. The reaction that the experiments follow is an
example of a crossed aldol condensation reaction where the intermediate in the
reaction becomes dehydrated to produce the resonance- stabilised unsaturated
ketone. The ketone loses a proton from an alpha-carbon to then form an enolate
ion, which attacks the carbonyl carbon of the aldehyde to yield, after protonation
alpha, beta-hydroxyketone. The intermediate formed for the aromatic aldehyde is
unstable and is subjected to base-catalysed dehydration, until the unsaturated
product is yielded. An important point to note is that a crossed-aldol condensation
leads to a number of different products, unless one of the carbonyl compounds
that is involved in the reaction cannot form an enolate ion (meaning compound
has no alpha-hydrogens). An aromatic aldehyde, such as Benzaldehyde is
sufficient for the reaction as only one enolate ion will form, which is from the other
carbonyl compound. Hence formation, the nucleophilic enolate ion attacks the
carbonyl carbon to form a β-hydroxycarbonyl product. The β-hydroxycarbonyl
product then eliminates a molecule of water to form a conjugated system
composed of a double bond and the carbonyl group. The elimination process with
regards to part one of the experiment was particularly fast as the alkene is
stabilised by conjugation to not only the carbonyl but also benzene. The
theoretical melting point of Dibenzalacetone is 110-111 °C. In theory, the melting
point of Dibenzalacetone is 55-57°C.
Reaction Mechanisms:
See post practical part (i) for reaction mechanisms
Experiment procedure
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
lOMoARcPSD|3377713
Part One – Synthesis of Dibenzalacetone.
Benzaldehyde (2.88g; 2.77mL) was added to a conical flask with acetone (0.79g;
1mL). The solution was shaken to ensure a homogeneous solution. Hence, 2M
sodium hydroxide (40mL) and ethanol (30mL) was added to a beaker. Half of the
solution from the conical flask was added to the beaker and mixed several times.
After 15 minutes, the remainder of the solution was added to the beaker. Ethanol
was used to ensure that all of the material had been transferred to the reaction
vessel. The reaction was timed for 30 minutes and hence the product was collected
by suction filtration. The solid that was collected was washed with water (3 x 60mL)
and then sucked as dry as possible. The product was then weighed using a scales
and the percentage yield before Recrystallisation was calculated. The
Dibenzalacetone was then recrystallized from ethanol (2.5mL/g). The product was
then dried in air and the percentage yield and melting point of the sample was
determined.
Part Two- Synthesis of Benzalacetophenone.
Benzaldehyde (2.88g; 2.77mL) was added to a conical flask with acetophenone
(3.26g; 3.17mL). The solution was mixed to ensure a homogeneous solution. Half of
the mixture was added to a beaker containing 2M sodium hydroxide (40mL) and
ethanol (30mL). The solution was swirled well and after 15 minutes the remainder of
the benzaldehyde/acetone mixture was added. Ethanol was used to transfer all the
solution to the reaction vessel. The solution was placed in ice. The reaction was
timed for 30 minutes, swirled occasionally. The solid was recrystallized. The product
was hence collected by suction filtration. Percentage yield and melting point was
determined.
Results
Part One:
Mr of acetone: 3(12.011)+ 6(1.0079) + 15.9994= 58.0798g.
Theoretical yield: 0.79g/ 58.0798g= 0.01360197521 moles.
Mr of Dibenzalacetone: 17(12.011)+ 14(1.0079) + 15.9994= 234.297g.
Amount of Dibenzalacetone Crystals obtained: 2.69g.
Actual yield: 2.69g/234.297g= 0.01148115426 moles.
Percentage yield before Recrystallization: Act/Theoretical:
0.01148115426/0.01360197521 x 100/1= 84.40799284%.
Recrystallization:
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
lOMoARcPSD|3377713
Crystals attained after Recrystallization: 2.69g- 1.47g= 1.22g.
Actual yield: 1.47g/234.297g=0.006274088016moles.
Theoretical Yield: 0.006274088016/0.01148115426 x 100/1= 54.64684015 is approx
equal to a 55% yield.
Melting point of Dibenzalacetone: 95 °C.
Part Two:
Mr of Benzaldehyde: 6(12.011) + 5(1.0079) + 12.011+ 1.0079+
15.9994=106.1238g.
Mr of Acetophenone: 8(12.011) + 8(1.0079) + 15.9994= 120.1506g.
1 x 2.88g/ 106.1238g= 0.02713811605 moles.
120.1506g x 0.02713811605= 3.260660926g
X= amount of Acetophenone required= 3.260660926g.
Density of Acetophenone= 1.03g/cm^3.
Amount required = 3.165690219 approx 3.17mL.
Discussion
In part one of the experiment the yield dramatically dropped after recrystallization. It
can be thought that this occurred for a number of reasons. One of which could
perhaps be that not all of the crystals were washed with ethanol and then transferred
into the filtration apparatus. Another could be that there was a number of impurities
present before recrystallization and hence some of these impurities were removed
after recrystallization. In addition, some of the product may have been lost due to
evaporation. Finally, some of the product could also have been lost during the
filtration process. The melting point was lower (95 °C) than the expected (110-111°C).
The lower melting point resulted as there was impurities present and impurities tend
to lower the melting point of compounds. In Part two of the experiment the limiting
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
lOMoARcPSD|3377713
reagent was found and its molar value and hence its molar value was multiplied by
the molecular mass of Acetophenone in order to calculate the value of x. The X
value was the amount of Acetophenone that was required to synthesis
Benzalacetophenone. The value of X was divided by the density of the ketone in
order to calculate the value in mL. The percentage yield and melting point for Part
Two was not calculated, due to the fact the experiment was not successful. The
experiment was not successful, due to not following procedures efficiently enough.
The mixture of Benzaldehyde and Acetophenone was added entirely to the NaOH
and ethanol, instead of in two aliquots (the second being after 15 minutes). Due to
not following the correct procedure Crystals did not form in the conical flask.
Perhaps, heating would have helped the solution to speed up the formation of
Crystals too.
Conclusions
Condensation reactions are reactions which join two or more molecules together,
usually with the loss of a small molecule such as water or an alcohol. Precaution
needs to be taken when following lab procedures, to ensure one obtains coherent
results. An enolate ion acts effectively as a nucleophile, which can be used to
synthesize Dibenzalacetone. Chalcone (Benzalacetophenone) is an important
aromatic ketone and enone that forms the central core in many biological
compounds.
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
lOMoARcPSD|3377713
Downloaded by Aizat aziz (aizat.aziz1997@gmail.com)
You might also like
- Reaction IntermediatesDocument12 pagesReaction IntermediatesJoya Bhatiagharu100% (3)
- Chm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetoneDocument17 pagesChm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetonesyafNo ratings yet
- PCP TekDocument1 pagePCP Tekgnarly tiredNo ratings yet
- Williamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsDocument8 pagesWilliamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsAnna LuthfiahNo ratings yet
- Leuckart ReactionDocument4 pagesLeuckart ReactionangelofgloryNo ratings yet
- 2021 Kinetics MCQ Quiz - Worked SolnsDocument3 pages2021 Kinetics MCQ Quiz - Worked SolnsPROgamer GTNo ratings yet
- Reductive Methylation of Primary and Secondary Amines and Amino Acids by Aqueous Formaldehyde and ZincDocument3 pagesReductive Methylation of Primary and Secondary Amines and Amino Acids by Aqueous Formaldehyde and Zincjavasolo100% (1)
- Illicit Drug Laboratories PDFDocument9 pagesIllicit Drug Laboratories PDFAlexander Melo AnguloNo ratings yet
- Benzyl Cyanide Hydrolysis To Acid - Big Chemical EncyclopediaDocument5 pagesBenzyl Cyanide Hydrolysis To Acid - Big Chemical EncyclopediaRon VoskNo ratings yet
- Cocaine For TropinoneDocument8 pagesCocaine For TropinoneJi ChemNo ratings yet
- P2P From Nutra Sweet - Ewok - Poacher - Downlowd Synthetikal - Org Sept (2007) PDFDocument3 pagesP2P From Nutra Sweet - Ewok - Poacher - Downlowd Synthetikal - Org Sept (2007) PDFdextroenantiomerNo ratings yet
- Synthesis of Methyl SalicylateDocument3 pagesSynthesis of Methyl SalicylateDike FahiraNo ratings yet
- Cataltic Applications of Metal CarbonylsDocument8 pagesCataltic Applications of Metal Carbonylssaud100% (4)
- Retrosynthetic Analysis PDFDocument6 pagesRetrosynthetic Analysis PDFHelmi FauziNo ratings yet
- Experiment 123456 1 PDFDocument18 pagesExperiment 123456 1 PDFHardi Ahmed100% (1)
- CyclohexeneDocument11 pagesCyclohexeneanon-407590100% (10)
- CHEM F110 - Lab Manual - Nov 5-2020Document45 pagesCHEM F110 - Lab Manual - Nov 5-2020STUTI MATHUR100% (2)
- ManualDocument8 pagesManualSweta Suman100% (1)
- Postlab-Grignard Reagent-Synthesis of TriphenylmethanolDocument8 pagesPostlab-Grignard Reagent-Synthesis of TriphenylmethanolMarleny ValenzuelaNo ratings yet
- 2 PyridonesDocument9 pages2 Pyridonesktraboulsi1No ratings yet
- Organic Chem Lab FDocument33 pagesOrganic Chem Lab FRanjith Kumar mNo ratings yet
- Synthesis of Acetophenone DerivativesDocument6 pagesSynthesis of Acetophenone DerivativesAwad SaidNo ratings yet
- Mandelic Acid: 1. ProcedureDocument4 pagesMandelic Acid: 1. ProcedureKangal PothikNo ratings yet
- Procedures For The Resolution of Racemic AmphetaminesDocument4 pagesProcedures For The Resolution of Racemic AmphetaminesЯн ДенисенкоNo ratings yet
- An Efficient Method For The Synthesis of 1,5-Benzodiazepine Derivatives Under Microwave Irradiation Without SolventDocument4 pagesAn Efficient Method For The Synthesis of 1,5-Benzodiazepine Derivatives Under Microwave Irradiation Without SolventHaouassi LakhdarNo ratings yet
- A-Bromination Using HBR H2O2 APKDocument7 pagesA-Bromination Using HBR H2O2 APKAshutosh BhaveNo ratings yet
- Claim and Ref of BenfotiamineDocument4 pagesClaim and Ref of BenfotiamineKanji PatelNo ratings yet
- Os Coll. Vol. 6 P175-PtabDocument5 pagesOs Coll. Vol. 6 P175-Ptabsunil_vaman_joshiNo ratings yet
- Synthesis of BenzocaineDocument4 pagesSynthesis of BenzocaineBebi TanNo ratings yet
- Process Optimization For Biosynthesis of PyruvateDocument19 pagesProcess Optimization For Biosynthesis of PyruvateGenceNo ratings yet
- Iodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsDocument6 pagesIodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsMike Roller100% (1)
- Kollidon-90-F Technical Information PDFDocument9 pagesKollidon-90-F Technical Information PDFPalak AgarwalNo ratings yet
- Synthesis of Acetyl Salicylic AcidDocument5 pagesSynthesis of Acetyl Salicylic AcidSilvia AryaniNo ratings yet
- Solvent Free Reduction of Aromatic Nitro Compounds With Alumina Supported Iron Powder and Acetic Acid Under Microwave IrradiationDocument5 pagesSolvent Free Reduction of Aromatic Nitro Compounds With Alumina Supported Iron Powder and Acetic Acid Under Microwave IrradiationKybernetikumNo ratings yet
- Octyl AcetateDocument6 pagesOctyl AcetateKristine BautistaNo ratings yet
- A Natural Decongestant: PseudoephedrineDocument1 pageA Natural Decongestant: PseudoephedrineGabriel NguyenNo ratings yet
- Aldol CondensationDocument3 pagesAldol CondensationDaniel McDermottNo ratings yet
- Hydriodic Acid - Step by Step Write-Up, Hive StimulantsDocument20 pagesHydriodic Acid - Step by Step Write-Up, Hive StimulantshenazoNo ratings yet
- Pseudoephedrine: 1. Synonyms CFR: Nist #Document18 pagesPseudoephedrine: 1. Synonyms CFR: Nist #Yuyun Saputri NingsihNo ratings yet
- Lloyd N. Ferguson - The Synthesis of Aromatic AldehydesDocument28 pagesLloyd N. Ferguson - The Synthesis of Aromatic AldehydesRoundSTICNo ratings yet
- MEDICINAL CHEMISTRY-I - Practicals PDFDocument25 pagesMEDICINAL CHEMISTRY-I - Practicals PDFAnit Dubey100% (1)
- Synthesis of Nitroalkanes From Bromoalkanes by Phase-Thansfer CatalysisDocument3 pagesSynthesis of Nitroalkanes From Bromoalkanes by Phase-Thansfer Catalysisscribd3822No ratings yet
- 9: Multistep Synthesis (Experiment) : Step 1: Synthesis of BenzoinDocument4 pages9: Multistep Synthesis (Experiment) : Step 1: Synthesis of BenzoinNaveed SajidNo ratings yet
- Itty BittyDocument6 pagesItty BittytroyNo ratings yet
- Reteta p2pDocument2 pagesReteta p2pJohn JohnNo ratings yet
- Friedel-Crafts AlkylationDocument7 pagesFriedel-Crafts AlkylationSalmaAlhasanNo ratings yet
- The NitroparaffinsDocument58 pagesThe NitroparaffinsKybernetikum100% (1)
- Lab Report 1 Synthesis and Characterization of Grignard ReagentDocument11 pagesLab Report 1 Synthesis and Characterization of Grignard ReagentFalak NazNo ratings yet
- The Reaction Between Sulfur and Calcium Hydroxide PDFDocument4 pagesThe Reaction Between Sulfur and Calcium Hydroxide PDFJavier Aviles100% (1)
- Red Phosphorousiodine Methamphetamine Synthesis - CompressDocument2 pagesRed Phosphorousiodine Methamphetamine Synthesis - Compressꜻⴆdồ éᴌρ̃̾ồρ̃̾No ratings yet
- Jamie P. Smith, Jonathan P. Metters, Osama I. G. Khreit, Oliver B. Sutcli Ffe, and Craig E. BanksDocument8 pagesJamie P. Smith, Jonathan P. Metters, Osama I. G. Khreit, Oliver B. Sutcli Ffe, and Craig E. BanksPastelituNo ratings yet
- Amino Acids With Secondary Amino Group5) (Reaction of Type 2) - (Akabori's Reaction)Document4 pagesAmino Acids With Secondary Amino Group5) (Reaction of Type 2) - (Akabori's Reaction)Doc MartenzNo ratings yet
- Synthesis of Bromobenzene: Required: Purified Benzene 34ml, Pyridine 0.5ml, Bromine, 24mlDocument2 pagesSynthesis of Bromobenzene: Required: Purified Benzene 34ml, Pyridine 0.5ml, Bromine, 24mljiskate77No ratings yet
- From Pepper (Piperonal) To MdaDocument14 pagesFrom Pepper (Piperonal) To MdaM. Shehryar KhanNo ratings yet
- Flaming Snowball Instruction & QuestionsDocument7 pagesFlaming Snowball Instruction & QuestionsJohn CenaNo ratings yet
- Hydrazine Formate ReductionsDocument3 pagesHydrazine Formate ReductionsPedro Mendonca100% (1)
- Pihkal: Chapter Nine Other Methods of Making PhenylacetoneDocument10 pagesPihkal: Chapter Nine Other Methods of Making Phenylacetones rNo ratings yet
- Synthetic Cannabinoids - Epidemiology, Pharmacodynamics, and Clinical Implications Nihms-2014Document61 pagesSynthetic Cannabinoids - Epidemiology, Pharmacodynamics, and Clinical Implications Nihms-2014smk0602No ratings yet
- Acetaminophen SynthesisDocument11 pagesAcetaminophen SynthesisCc BpumpNo ratings yet
- Exer 9b Post Lab ReportDocument6 pagesExer 9b Post Lab ReportKatrinne Clea PincaNo ratings yet
- Pre LabghvyicDocument6 pagesPre LabghvyicGobe JamNo ratings yet
- Chem 305 Lab OneDocument6 pagesChem 305 Lab OneGobe JamNo ratings yet
- Theory of The Aldol Condensation ReactionDocument9 pagesTheory of The Aldol Condensation ReactionaizatNo ratings yet
- Experiment With The First ClassDocument2 pagesExperiment With The First ClassaizatNo ratings yet
- Experiment 2 Sodium BorohydrideDocument2 pagesExperiment 2 Sodium BorohydrideaizatNo ratings yet
- Synthesis of VanillinDocument2 pagesSynthesis of VanillinaizatNo ratings yet
- CHM556 Organic Chemistry Ii Laboratory: The TechniquesDocument3 pagesCHM556 Organic Chemistry Ii Laboratory: The TechniquesaizatNo ratings yet
- POC-1, University Question PaperDocument8 pagesPOC-1, University Question Papersatheeshpharma6No ratings yet
- Chapter 9Document16 pagesChapter 9Joseph Ken AlcalaNo ratings yet
- Chapter 2 Chemical KineticsDocument83 pagesChapter 2 Chemical KineticsRaymond KakalaNo ratings yet
- Chemical KineticsDocument72 pagesChemical KineticsSiddhartha KumarNo ratings yet
- q1 Module 10Document15 pagesq1 Module 10Princess Angeles Andam100% (1)
- Benzene WorksheetDocument7 pagesBenzene WorksheetTanzimNo ratings yet
- Reactants Products: Double Lined Boxes Are Conversion Factors To Convert From One Quantity To AnotherDocument5 pagesReactants Products: Double Lined Boxes Are Conversion Factors To Convert From One Quantity To AnotherfaltriqueraNo ratings yet
- Acetato de Etilo GPSDocument40 pagesAcetato de Etilo GPSDaniel Camilo CanoNo ratings yet
- Interpretation of Batch Reactor Data: Chapter ThreeDocument45 pagesInterpretation of Batch Reactor Data: Chapter ThreeAnnisa RizqiaNo ratings yet
- Chapter 9-Alkynes 7 Unsaturations 9 Unsaturations 5 UnsaturationsDocument19 pagesChapter 9-Alkynes 7 Unsaturations 9 Unsaturations 5 Unsaturations張湧浩No ratings yet
- Chem 305 Lab OneDocument6 pagesChem 305 Lab OneGobe JamNo ratings yet
- Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic ApplicationsDocument58 pagesOrganocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic ApplicationschidambaramrNo ratings yet
- Enzymes IGCSE 2023Document11 pagesEnzymes IGCSE 2023chiwooutlook.com.jNo ratings yet
- Chapter 1 Form 5 Chemistry 2015Document21 pagesChapter 1 Form 5 Chemistry 2015Alvieno Situl MintowNo ratings yet
- Addition ReactionsDocument54 pagesAddition ReactionsRithvik ShastryNo ratings yet
- Chemistry 18 - Notes PDFDocument63 pagesChemistry 18 - Notes PDFGeorge Miguel Obusán100% (2)
- Substitution Reactions (S 2 Versus S 1) : Chemistry 5.12 Spring 2003, Handout #9Document2 pagesSubstitution Reactions (S 2 Versus S 1) : Chemistry 5.12 Spring 2003, Handout #9amrhkmhNo ratings yet
- bài tập cuối kỳDocument14 pagesbài tập cuối kỳKHÁNH VÕ ĐĂNGNo ratings yet
- Simple Collision TheoryDocument30 pagesSimple Collision TheoryJona FranciscoNo ratings yet
- Advanced CatalysisDocument24 pagesAdvanced CatalysisSuviti ChariNo ratings yet
- Chemical Kinetics: Matching Answer Type QuestionsDocument5 pagesChemical Kinetics: Matching Answer Type QuestionsAbhiNo ratings yet
- Chapter 1Document60 pagesChapter 1Janagaraj SukumaranNo ratings yet
- Arynes in Natural Product SynthesisDocument14 pagesArynes in Natural Product SynthesisIJRASETPublicationsNo ratings yet
- Chemical Equilibrium: Ajay Kumar Panigrahi, M.Sc. in Organic Chemistry, NIT WarangalDocument19 pagesChemical Equilibrium: Ajay Kumar Panigrahi, M.Sc. in Organic Chemistry, NIT WarangalAjay Kumar PanigrahiNo ratings yet
- Sample Questions - Chapter 16Document8 pagesSample Questions - Chapter 16Rasel Islam100% (1)
- Introduction To Rates of ReactionDocument14 pagesIntroduction To Rates of Reactionash4evaNo ratings yet