Professional Documents
Culture Documents
Hubungan Konsentrasi Dengan Laju Reaksi: Urutan: Pati, Na2S2O5, Hgcl2, Kio3
Hubungan Konsentrasi Dengan Laju Reaksi: Urutan: Pati, Na2S2O5, Hgcl2, Kio3
Uploaded by
Inggar Clalu Ingin BersamanyaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hubungan Konsentrasi Dengan Laju Reaksi: Urutan: Pati, Na2S2O5, Hgcl2, Kio3
Hubungan Konsentrasi Dengan Laju Reaksi: Urutan: Pati, Na2S2O5, Hgcl2, Kio3
Uploaded by
Inggar Clalu Ingin BersamanyaCopyright:
Available Formats
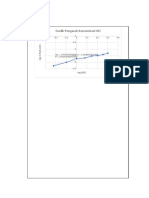
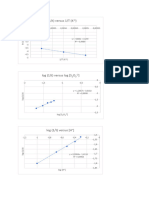
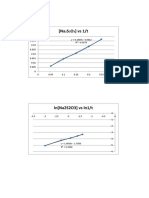
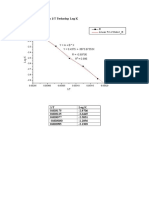
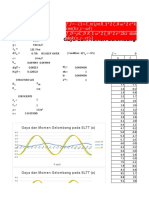
Hubungan Konsentrasi dengan Laju Reaksi -1.
777
-1.75
-1.21 -1.205 -1.2 -1.195 -1.19 -1.185 -1.18 -1.175 -1.17
-1.8
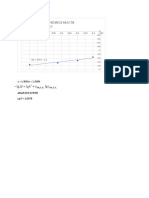
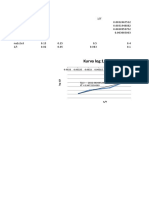
y = 8.3459x + 7.9876
R² = 0.8783 -1.85
-1.9
log v (M/S)
-1.95
-2.03 -2
-2.05
-2.05
-2.1
-2.15
log M(M)
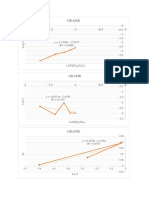
Urutan: Pati, Na2S2O5, HgCl2, KIO3
Urutan: Pati, Na2S2O5, HgCl2, KIO3
You might also like
- Grafik p4Document3 pagesGrafik p4Baba BubuNo ratings yet
- LN K vs. 1/T (In Kelvin)Document2 pagesLN K vs. 1/T (In Kelvin)Remjohn MagtaasNo ratings yet
- Grafik JurnalDocument1 pageGrafik JurnalHusna SulistiawatiNo ratings yet
- Kimia P2 P4Document2 pagesKimia P2 P4Salsabila SalsabilaNo ratings yet
- LPRN 2Document4 pagesLPRN 2Isna DewiyantiNo ratings yet
- Gráficos - Lab de QuímicaDocument1 pageGráficos - Lab de QuímicaBruno RitondaroNo ratings yet
- Graph Order of Na S ODocument2 pagesGraph Order of Na S OAhlan RiwahyuNo ratings yet
- Question 14Document3 pagesQuestion 14emmanuel limaNo ratings yet
- Ingenieria de La ReaccionesDocument21 pagesIngenieria de La ReaccionesManuela Ospina ArboledaNo ratings yet
- Bab IiDocument2 pagesBab IiandhikaNo ratings yet
- Lampiran p2Document2 pagesLampiran p2shafiyahsalsabila51No ratings yet
- DGDSGSDDocument2 pagesDGDSGSDTri Pindi HandayaniNo ratings yet
- Logx/M vs. Logc Logx/M vs. LogcDocument2 pagesLogx/M vs. Logc Logx/M vs. LogcZhu Chen ChuanNo ratings yet
- Examen Final de Analisis Estructural Ii - PfaDocument3 pagesExamen Final de Analisis Estructural Ii - PfaPercy Eduardo León CubasNo ratings yet
- Grafik Hubungan Laju Reaksi Dengan Konsentrasi HCLDocument2 pagesGrafik Hubungan Laju Reaksi Dengan Konsentrasi HCLBhakti RahmadhaniNo ratings yet
- Grafik Hubungan Antara 1/T Terhadap Log K: B Linear Fit of Data1 - BDocument1 pageGrafik Hubungan Antara 1/T Terhadap Log K: B Linear Fit of Data1 - BAwaliyatun Fhathonatuz ZuhriyahNo ratings yet
- E1Document11 pagesE1Boyce NgomaNo ratings yet
- Log 1/EC50: Data QsarDocument4 pagesLog 1/EC50: Data QsarAyyu WidyazmaraNo ratings yet
- Libro 1Document4 pagesLibro 1Fabian Alexander Nova MendozaNo ratings yet
- New Microsoft Excel WorksheetDocument10 pagesNew Microsoft Excel WorksheetSoumaia HaidarNo ratings yet
- C) Statistical Empirical Findings: Statistic - HTMDocument3 pagesC) Statistical Empirical Findings: Statistic - HTMalexisgarefalakisNo ratings yet
- Graph f2 PDFDocument3 pagesGraph f2 PDFnoraNo ratings yet
- Hydraulic Gradient, I Vs Velocity, V (M/S)Document3 pagesHydraulic Gradient, I Vs Velocity, V (M/S)noraNo ratings yet
- Reaction Order Na S O: GraphicDocument3 pagesReaction Order Na S O: GraphicAlvira DwiNo ratings yet
- Gaya Dan Momen Gelombang Pada SLTT (A)Document22 pagesGaya Dan Momen Gelombang Pada SLTT (A)kometmayorNo ratings yet
- Ordin de Reactie LGC: C A K TDocument1 pageOrdin de Reactie LGC: C A K TRobert BarbuNo ratings yet
- 49-SA16145S-A01A - 3 - Annex Soil Test Results PDFDocument11 pages49-SA16145S-A01A - 3 - Annex Soil Test Results PDFZhao QinglongNo ratings yet
- Grafik Isoterm AdsorpsiDocument2 pagesGrafik Isoterm AdsorpsiRezky OkfaistellaNo ratings yet
- Bending Moment and Shear Force Diagram - Cantilever Both Ends Sse-Excel SectionDocument1 pageBending Moment and Shear Force Diagram - Cantilever Both Ends Sse-Excel SectionSES DESIGNNo ratings yet
- Kurva Log 1/t Vs 1/TDocument3 pagesKurva Log 1/t Vs 1/TBadzlinaKhairunizzahraNo ratings yet
- Gaya Dan Momen Gelombang Pada SLTT (A)Document20 pagesGaya Dan Momen Gelombang Pada SLTT (A)kometmayorNo ratings yet
- The Rate of Grafting and Some Kinetic Parameters of The Graft Copolymerization of Methacrylic Acid On Poly (Ethylene Terephthalate) Fibers With Azobisisobutyronitrile (#143031) - 124454Document6 pagesThe Rate of Grafting and Some Kinetic Parameters of The Graft Copolymerization of Methacrylic Acid On Poly (Ethylene Terephthalate) Fibers With Azobisisobutyronitrile (#143031) - 124454Francisco AdalbertoNo ratings yet
- Tugas 01Document6 pagesTugas 01Nur IkhsanudinNo ratings yet
- Book 1Document2 pagesBook 1maduNo ratings yet
- Book1 1Document2 pagesBook1 1AndiniNo ratings yet
- CE257 Data Communication and Networking: By: Dr. Ritesh Patel Ce Dept, Cspit, Charusat Riteshpatel - Ce@charusat - Ac.inDocument77 pagesCE257 Data Communication and Networking: By: Dr. Ritesh Patel Ce Dept, Cspit, Charusat Riteshpatel - Ce@charusat - Ac.inDHRUVNo ratings yet
- Experiment Write Up Rate of Change of MomentumDocument1 pageExperiment Write Up Rate of Change of MomentumM IKNo ratings yet
- Static Moment Sse-Excel Section: The Author Will Not Be Responsible For Any Uses of This Software! v1.06Document1 pageStatic Moment Sse-Excel Section: The Author Will Not Be Responsible For Any Uses of This Software! v1.06SES DESIGNNo ratings yet
- Plot Log (H+) Vs Log 1/trata-RataDocument1 pagePlot Log (H+) Vs Log 1/trata-RataRahma DaniatyNo ratings yet
- Grafik Isoterm Adsorpsi Larutan CH3COOH Terhadap Karbon AktifDocument3 pagesGrafik Isoterm Adsorpsi Larutan CH3COOH Terhadap Karbon AktifAdinda SaraswatiNo ratings yet
- Pourbaix DiagramDocument10 pagesPourbaix DiagramForrest PommierNo ratings yet
- Crack Growth Curve: Number of CyclesDocument5 pagesCrack Growth Curve: Number of CyclesGundagani Sai Kumar ce18m081No ratings yet
- Excel (Student)Document2 pagesExcel (Student)dhinesh gNo ratings yet
- CorrosiónDocument2 pagesCorrosiónEnrique_Antonio_80No ratings yet
- Valores Orientadores EPADocument87 pagesValores Orientadores EPAJailson SilvaNo ratings yet
- LPRNDocument4 pagesLPRNIsna DewiyantiNo ratings yet
- Column ReportDocument45 pagesColumn ReportOkechukwu BenjaminNo ratings yet
- Combinations of Loads (ACI 95) : Load CasesDocument1 pageCombinations of Loads (ACI 95) : Load Casesthongchai_007No ratings yet
- Temperatu Pressure 700 1.007902 5 Gas/Co 900 1.208165 5 Sio2 (Quart 1100 1.861712 5 1300 1.990051 5 1500 1.998716 5Document3 pagesTemperatu Pressure 700 1.007902 5 Gas/Co 900 1.208165 5 Sio2 (Quart 1100 1.861712 5 1300 1.990051 5 1500 1.998716 5Bùi Hắc HảiNo ratings yet
- CementacionDocument3 pagesCementacionDiego RoqueNo ratings yet
- Keterangan: Titrasi Ke 1 2 Volume Awal 5,2 5,5 Volume Akhir 5,5 5,9 Volume Titrasi 0,3 0,4Document2 pagesKeterangan: Titrasi Ke 1 2 Volume Awal 5,2 5,5 Volume Akhir 5,5 5,9 Volume Titrasi 0,3 0,4Dina Aulya SeptianiNo ratings yet
- Asignacion 10 Andrea ChavezDocument7 pagesAsignacion 10 Andrea ChavezKEVIN ALEXIS FLORES VERDUGONo ratings yet
- Concentración Vs TiempoDocument4 pagesConcentración Vs TiempoSamie ReyNo ratings yet
- Orden de Reacción Del Ión Fe3+Document5 pagesOrden de Reacción Del Ión Fe3+Rafael GonzálezNo ratings yet
- Tugas2 - Half-Lives Method - K4Document3 pagesTugas2 - Half-Lives Method - K4Anas GhozaliNo ratings yet
- FCG CalculationDocument3 pagesFCG Calculationkarthip08No ratings yet
- Cross in Cadru Din Incarcare DistribuitaDocument8 pagesCross in Cadru Din Incarcare DistribuitaZgripcea CristianNo ratings yet
- Determination of Specific Rate Constant of ReactionDocument6 pagesDetermination of Specific Rate Constant of ReactionRafid JawadNo ratings yet
- Grafik OrdeDocument1 pageGrafik OrdeInggar Clalu Ingin BersamanyaNo ratings yet
- Grafik OrdeuDocument5 pagesGrafik OrdeuInggar Clalu Ingin BersamanyaNo ratings yet
- Grafik OrdeDocument1 pageGrafik OrdeInggar Clalu Ingin BersamanyaNo ratings yet
- Materials Letters: Seungsun Lee, Young-Min ChungDocument4 pagesMaterials Letters: Seungsun Lee, Young-Min ChungInggar Clalu Ingin BersamanyaNo ratings yet