Professional Documents
Culture Documents
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
Uploaded by
ravi2likeCopyright:
Available Formats
You might also like
- Paper Trade TJS Elite v8.9.2 - S-O REVISEDDocument1,170 pagesPaper Trade TJS Elite v8.9.2 - S-O REVISEDBryan Dawi42% (12)
- 9autacoids 14010v9023022 Phpapp02Document109 pages9autacoids 14010v9023022 Phpapp02h3cn1r3100% (1)
- IRDAI Memorandum Jeevan Saral Final DraftDocument4 pagesIRDAI Memorandum Jeevan Saral Final DraftMoneylife FoundationNo ratings yet
- Histamine and AntihistaminesDocument26 pagesHistamine and Antihistaminesgloria anyebeNo ratings yet
- Autacoids: Group No. 1Document92 pagesAutacoids: Group No. 1Rohan Pal100% (1)
- Histamine and AntihistaminesDocument26 pagesHistamine and Antihistaminesgloria anyebeNo ratings yet
- Autacoids Final PDFDocument22 pagesAutacoids Final PDFIqra ShaikhNo ratings yet
- Antimicrobial Agents-NrsDocument24 pagesAntimicrobial Agents-NrsSusmita BeheraNo ratings yet
- Autacoids A4 56Document2 pagesAutacoids A4 56ahmedsalah565vvvNo ratings yet
- AutacoidsDocument39 pagesAutacoidszeyadthemedicNo ratings yet
- Histamine and Serotonin: by Srota Dawn. M - Pharma (Pharmacology)Document34 pagesHistamine and Serotonin: by Srota Dawn. M - Pharma (Pharmacology)Rosel SorianoNo ratings yet
- Histamine Ans Serotonin and Their Antagonists 2020 - 2021Document109 pagesHistamine Ans Serotonin and Their Antagonists 2020 - 2021Sil Ana Salissou AbdouNo ratings yet
- Autacoids For Med.Document140 pagesAutacoids For Med.Feysal AhmedNo ratings yet
- 10 - AutacoidsDocument67 pages10 - AutacoidscchatrumaNo ratings yet
- Histamine: Dr. Anil Kumar SaxenaDocument17 pagesHistamine: Dr. Anil Kumar SaxenaLuqman Al-Bashir FauziNo ratings yet
- Autocoids, Histamine and Antihistaminic-Dr - Jibachha Sah, M.V.SC, LecturerDocument27 pagesAutocoids, Histamine and Antihistaminic-Dr - Jibachha Sah, M.V.SC, Lecturerjibachha sahNo ratings yet
- Histamine ..Document30 pagesHistamine ..Sunanda mohanNo ratings yet
- Pheromones, AutacoidsDocument64 pagesPheromones, Autacoidskelvinmaina9993No ratings yet
- AutacoidsDocument43 pagesAutacoidsOdiete EfeNo ratings yet
- Local Media1236679140 PDFDocument12 pagesLocal Media1236679140 PDFNusrat JahanNo ratings yet
- Autacoid Lec 1 & 2Document45 pagesAutacoid Lec 1 & 2s.nilormee1201No ratings yet
- Clinical Science SessionDocument12 pagesClinical Science SessionSelvi Puspa SariNo ratings yet
- AntiAllergy and Immunosupresive DrugDocument51 pagesAntiAllergy and Immunosupresive Drugfachrie saputraNo ratings yet
- Farmakologi Antihistamin-RDY 2019Document50 pagesFarmakologi Antihistamin-RDY 2019fandi mnNo ratings yet
- Anti His Tami NicDocument24 pagesAnti His Tami Nicgoharianali3000No ratings yet
- Antihistamine Presentation-KhallDocument34 pagesAntihistamine Presentation-Khallmaxwell amponsahNo ratings yet
- AUTOCOIDS - HISTAMINESDocument7 pagesAUTOCOIDS - HISTAMINESneonoelsmkNo ratings yet
- Autacoids (Local Hormones) and Their Pharmacolo-Gical ModulationDocument75 pagesAutacoids (Local Hormones) and Their Pharmacolo-Gical ModulationAgung PutraNo ratings yet
- Autacoids Related DrugsDocument74 pagesAutacoids Related DrugsVikash KushwahaNo ratings yet
- HISTAMINEDocument29 pagesHISTAMINEShehu Baba AbdullahiNo ratings yet
- AutacoidsDocument124 pagesAutacoidsMuhammad Modu BulamaNo ratings yet
- Histamine and Anti HistaminesDocument12 pagesHistamine and Anti HistaminesshrikantNo ratings yet
- AntihistaminDocument46 pagesAntihistaminVenerandaNo ratings yet
- HistamineDocument11 pagesHistamineOoi Ah GuanNo ratings yet
- Bp503t Pcol Unit-IIIDocument38 pagesBp503t Pcol Unit-IIIAakkkNo ratings yet
- Histamine & Antihistaminics: DR - Vijay BhushanamDocument24 pagesHistamine & Antihistaminics: DR - Vijay BhushanamTazrian FarahNo ratings yet
- 4 AutacoidsDocument9 pages4 AutacoidsAli EllaffiNo ratings yet
- AUTACOIDSDocument7 pagesAUTACOIDSElijah ChiumyaNo ratings yet
- DR Tehniat ZahraDocument19 pagesDR Tehniat ZahraRphTehniat ZahraNo ratings yet
- Pharma 24 To 31Document233 pagesPharma 24 To 31Loai Mohammed IssaNo ratings yet
- Antihistamine Presentation KhallDocument34 pagesAntihistamine Presentation KhallAdam Aulia RahmanNo ratings yet
- AntihistamineDocument2 pagesAntihistamineMichelle CasilangNo ratings yet
- AutacoidsDocument14 pagesAutacoidsMahmoud AboudNo ratings yet
- Histamine Pharmacology 2024Document24 pagesHistamine Pharmacology 2024Sylvester AsareNo ratings yet
- Slides AUTACOIDSDocument29 pagesSlides AUTACOIDSStrange eeNo ratings yet
- Autacoids: AntagonistsDocument37 pagesAutacoids: AntagonistsJayaNo ratings yet
- AntihistaminDocument46 pagesAntihistaminVenerandaNo ratings yet
- Histamine and Antihistamines LatestDocument28 pagesHistamine and Antihistamines LatestAjay KumarNo ratings yet
- HistamineDocument16 pagesHistamineسویرا منیرNo ratings yet
- Week 12 - 13 LongDocument62 pagesWeek 12 - 13 LongTom perryNo ratings yet
- Katzung - AutacoidsDocument23 pagesKatzung - AutacoidsKhairul AimanNo ratings yet
- With Important Actions On Smooth MuscleDocument90 pagesWith Important Actions On Smooth MuscleGeraldine Marie Salvo100% (1)
- Presentation 1Document25 pagesPresentation 1christinaNo ratings yet
- HistamineDocument14 pagesHistamineArslan RajpootNo ratings yet
- 1 Inflammation - Allergy - 230522 - 113129Document58 pages1 Inflammation - Allergy - 230522 - 113129Csparkz beautyNo ratings yet
- Kimia Farmasi: AntihistaminDocument25 pagesKimia Farmasi: AntihistaminchristinaNo ratings yet
- Autocoids 2Document68 pagesAutocoids 2Maryam NisaNo ratings yet
- Lipoproteins: - Function: Transport of Fat Soluble Substances - Types: 1) Chylomicron 2) VLDL 3) LDL 4) HDLDocument31 pagesLipoproteins: - Function: Transport of Fat Soluble Substances - Types: 1) Chylomicron 2) VLDL 3) LDL 4) HDLRexel EnnyNo ratings yet
- AntihistaminDocument44 pagesAntihistaminDWI RAHMA HALIDANo ratings yet
- Low Histamine Cooking in Your Instant Pot: 75 Easy Meals for Everyday HealingFrom EverandLow Histamine Cooking in Your Instant Pot: 75 Easy Meals for Everyday HealingNo ratings yet
- Pharm 3 IDocument3 pagesPharm 3 Iravi2like100% (1)
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 4 CDocument4 pagesPharm 4 Cravi2likeNo ratings yet
- Pharm 4 ADocument3 pagesPharm 4 Aravi2likeNo ratings yet
- Pharm 3 BDocument3 pagesPharm 3 Bravi2likeNo ratings yet
- By Duy Thai, 1997: Choline AcetyltransferaseDocument4 pagesBy Duy Thai, 1997: Choline Acetyltransferaseravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 3 eDocument3 pagesPharm 3 eravi2likeNo ratings yet
- Pharm 3 CDocument3 pagesPharm 3 Cravi2likeNo ratings yet
- By Duy Thai, 1997: Diffuse Out of Nerve Terminal and Act On COMPT in External TissuesDocument5 pagesBy Duy Thai, 1997: Diffuse Out of Nerve Terminal and Act On COMPT in External Tissuesravi2likeNo ratings yet
- Anti Inflammatory Drugs, by Duy Thai, 1997Document4 pagesAnti Inflammatory Drugs, by Duy Thai, 1997ghinsavitNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2like100% (2)
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 1 HDocument6 pagesPharm 1 Hravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 5Document5 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 5ravi2likeNo ratings yet
- Stimulates Ca ") .: 1997 Pharmacology Semester 1 Page 1 of 6Document6 pagesStimulates Ca ") .: 1997 Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- 1997 Pharmacology Semester 1 Page 1 of 6Document6 pages1997 Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- E Valency Ion Ion: A - Ve Value Means A Net Flow Into The CellDocument5 pagesE Valency Ion Ion: A - Ve Value Means A Net Flow Into The Cellravi2likeNo ratings yet
- Aspirin Irreversibly Inhibits Cyclo-Oxygenase: Pharmacology Semester 1 Page 1 of 5Document6 pagesAspirin Irreversibly Inhibits Cyclo-Oxygenase: Pharmacology Semester 1 Page 1 of 5ravi2likeNo ratings yet
- Pharm 1 GDocument3 pagesPharm 1 Gravi2likeNo ratings yet
- Pharm 1 eDocument3 pagesPharm 1 eravi2likeNo ratings yet
- Presence of An Antagonist.: Pharmacology Semester 1 Page 1 of 5Document5 pagesPresence of An Antagonist.: Pharmacology Semester 1 Page 1 of 5ravi2like100% (1)
- 1997 Pharmacology Semester 1 Page 1 of 4Document4 pages1997 Pharmacology Semester 1 Page 1 of 4ravi2like100% (1)
- Pharm 1 ADocument3 pagesPharm 1 Aravi2likeNo ratings yet
- Kelim Rate of Drug Elimination From The Plasma T Time: Pharmacology Semester 1 Page 1 of 6Document6 pagesKelim Rate of Drug Elimination From The Plasma T Time: Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- 1997 Pharmacology Semester 1 Page 1 of 7Document7 pages1997 Pharmacology Semester 1 Page 1 of 7ravi2likeNo ratings yet
- Time Value of Money: Compound InterestDocument28 pagesTime Value of Money: Compound InterestkateNo ratings yet
- Cad Notes PDFDocument61 pagesCad Notes PDFSuresh Natarajan100% (3)
- Bus FestoDocument82 pagesBus FestoraphvalonNo ratings yet
- Writing Research Papers James D Lester PDFDocument4 pagesWriting Research Papers James D Lester PDFgahebak1mez2100% (1)
- Inv - 1124003027 - Po - 407097662 - VR - 407097662 - (Top Synthetic Rubber)Document1 pageInv - 1124003027 - Po - 407097662 - VR - 407097662 - (Top Synthetic Rubber)shamirah98No ratings yet
- Applied Sciences: Determination of PH in Powdered Concrete Samples or in SuspensionDocument10 pagesApplied Sciences: Determination of PH in Powdered Concrete Samples or in SuspensionKarthick MNo ratings yet
- Bar Charts in ResearchDocument5 pagesBar Charts in ResearchPraise NehumambiNo ratings yet
- New MONDDocument7 pagesNew MONDVernonNo ratings yet
- WITH SOLUTIONS Pipe Connection and Three ReservoirDocument46 pagesWITH SOLUTIONS Pipe Connection and Three ReservoirMark PulongbaritNo ratings yet
- Lec 3Document32 pagesLec 3wjeelaniNo ratings yet
- B.Sc. SEM IDocument4 pagesB.Sc. SEM IsameerNo ratings yet
- Ghirri - Redutores - Classificação - Um-Vs-Rev.20.06.06-Eng3Document35 pagesGhirri - Redutores - Classificação - Um-Vs-Rev.20.06.06-Eng3Jeferson DantasNo ratings yet
- ScrewCompressors XRV204 SpecSheet DigitalDocument1 pageScrewCompressors XRV204 SpecSheet DigitalBarros Arias EnriqueNo ratings yet
- Home Learning IB GR 12 June 4, 7 - 11Document6 pagesHome Learning IB GR 12 June 4, 7 - 11Arnel Ordas AvilaNo ratings yet
- Phrasal Verb LESSON FINAL 1701874327225Document67 pagesPhrasal Verb LESSON FINAL 1701874327225bgt dfgNo ratings yet
- LPP - orDocument12 pagesLPP - orbharat_v79No ratings yet
- LLM Placement Brochure 2021 22 V15Document24 pagesLLM Placement Brochure 2021 22 V15Bala KumaranNo ratings yet
- Sarah Tchoukaleff ResumeDocument1 pageSarah Tchoukaleff Resumeapi-261081956No ratings yet
- Ad 345Document1 pageAd 345Vojtech ChalupaNo ratings yet
- 2008 Bullentin Rel. 2-DataDocument5 pages2008 Bullentin Rel. 2-DataKalai SelvanNo ratings yet
- Valuecent Profile PDFDocument9 pagesValuecent Profile PDFAP Advisory BVNo ratings yet
- Individual Taxpayers Tax Filing ExercisesDocument3 pagesIndividual Taxpayers Tax Filing ExercisesKIM RAGANo ratings yet
- Workout ChartDocument1 pageWorkout ChartAsadullah JawaidNo ratings yet
- BELOW KNEE AMPUTATION EditDocument16 pagesBELOW KNEE AMPUTATION EditPutri AyuNo ratings yet
- 2.1 Theory of Metal Cutting Q&A For StudentDocument8 pages2.1 Theory of Metal Cutting Q&A For Studentnikhilbatham0% (1)
- TIFDDocument1 pageTIFDAtulNo ratings yet
- Rural Development: 14.1. Rural Scenario in Tamil NaduDocument17 pagesRural Development: 14.1. Rural Scenario in Tamil NaduhinduNo ratings yet
- 8 SWOT Analysis Tools For Small Businesses: 1. SmartsheetDocument3 pages8 SWOT Analysis Tools For Small Businesses: 1. SmartsheetSantu BiswaaNo ratings yet
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
Uploaded by
ravi2likeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 6
Uploaded by
ravi2likeCopyright:
Available Formats
By Duy Thai, 1997 Pharmacology Semester 1 page 1 of 6
HISTAMINE AND ANTIHISTAMINES
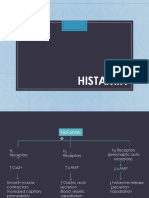
Synthesis and metabolism of histamine
Histidine
(From diet)
L histidine decarboxylase
Histamine N Histamine
methyl transferase Diamine oxidase
(DAO)
N methyl histamine Imidazole acetic
acid (IAA)
Monoamine oxidase
(MAO)
N methylimidazole acetic
IAA Riboside
acid (NMAA)
If histamine is
overproduced, NMAA
will be the diagnostic
Excreted in urine
marker in the urine
Distribution, turnover and storage of histmine
• In the tissues, the histamine concentration can range from less than 1 to over 100µg/g
• The tissue distribution of histamine is identical to the mast cell distribution (wherever mast cells are, there
will most likeley be histamine present).
• Mast cells are distributed in:
• Skin
• Bronchial mucosa
• Gut mucosa
• Other mucosal surfaces
• Mast cells synthesis histamine very slowly and store it in specific granules for a very long time (until
needed).
• Histamine levels in the plasma are normally very low.
• Histamine levels in the CSF are high.
• In the brain, stomach, healing tissues and some cells in the skin, histamine is not stored in granules.
• It is produced de novo
• It has a high turnover (made quickly, destroyed quickly)
• Low steady state levels
Basic mast cell biology
• An allergic response will occur when an allergen causes cross linking of IgE molecules.
• The cross linking of IgE antibodies will cause the IgE receptors (Fcε R1) on the surface of the mast cell to be
brought into close proximity to each other.
• The Fcε R1 receptor has an intracellular domain which belongs to the src family of kinases. The kinases are tyrosine
kinases, of which there are 2 (lyn and syk). When IgE binds, it casues a conformational change which allows
phosphorylation of one of these tyrosine residues (lets say lyn – it may be syk).
• Phosphorylation of the tyrosine activates the tyrosine kinase (lyn). This activated tyrosine kinase cross
phosphorylates the other tyrosine kinase (syk) on the other Fcε R1 receptor.
• The phosphorylation of the syk tyrosine kinases allows phosphorylates the enzymes phospholipase C.
• Note that for those who do not get allergic reactions, there is no cross linking of IgE molecules and so only
one Fcε R1 receptor is activated. The binding of IgE to the receptor will cause phosphorylation of the lyn
By Duy Thai, 1997 Pharmacology Semester 1 page 2 of 6
tyrosine kinase but since there is no other Fcε R1 nearby, cross phosphorylation of syk cannot occur. It is
the syk tyrosine kinase which is able to activate phospholipase C.
• PLC breaks down membrane phospholipids and releases IP3 as a product.
• IP3 causes the release of Ca2+ from intracellular stores.
• Ca2+ results in the release of granules (Ca2+ dependent exocytosis)
• The stored granules have a pH of 5.5. At this pH histamine is positively charged. It is held inside the
granule by ionic interaction with negatively charged molecules also in the granule.
• These molecules are:
• Heparin
• Chondroitin sulphate
• Proteases
• The histamine released can act on the mast cell it came from and inhibit the further release of histamine
(autoinhibition). Histamine does this by acting on a H2 receptor on the mast cell.
• The H2 receptor causes the activation of adenylate cyclase which increases the amount of cAMP.
• cAMP inhibits the release of histamine
• The mast cell also has a β2 receptor which also does the same thing. Therefore, β2 antagonists can inhibit the
release of histamine from mast cells.
What triggeres the release of histamine?
• Anaphylactic triggers
• Antigen specific, IgE mediated
• Anaphylactoid triggers
• Organic bases Many drugs used in therapy
• Morphine can trigger mast cells to
• Tubocuravine release histamine
Neuromuscular blockers
• Succinylchloride
• Contrast media used in radiography
• Dextran
• Vancomycin (an antibiotic)
• Complement (C5a, C3a)
• Some neuropeptides
• Venom from insects (e.g. mastoparan from wasps)
Diseases associated with increased histamine
• Urticaria pigmentosa (mastocytosis)
• Increased mast cells in skin
By Duy Thai, 1997 Pharmacology Semester 1 page 3 of 6
• Itchy, coloured lesions
• Systemic mastocytosis
• Mast cells increase in organs
• Myelogenous leukemia
• Increased numbers of basophils
• Basophils are of different origin to mast cells but have similar effects
• Gastrinoma
• Zollinger-Ellison syndrome
• Increase in gastrin release resulting in excessive release of histamine from ECL cells in the gut.
• Get ulcers and diarrhea.
• Urticaria
• Dermographism
• Puritis
• Headaches
• Weakness All these can be caused by histamine
• Hypotension
• Flushing of the skin
• GIT disturbances
• Ulcers
Effects of histamine in humans
• Burning Due to sensory nerve stimulation
• Itching Due to senosry nerve stimulation
• Warmth Due to sensory nerve stimulation/vasodilation
• Skin flush Vasodilation
• Hypotension Vasodilaton which leads to reduced peripheral resistance
• Increased HR Reflex tachycardia to compensate for the reduction in blood pressure.
• Headache Vasodilation/stimulation of afferent nerve fibres
• Skin oedema/hives Microvascular leakage
• Colic/nausea GI irritation
• Acid hypersecretion Action of histamine on H2 receptors on partietal cells
• Bronchospasm Effects of histamine on smooth muscle contraction in bronchi
• Triple response
• Red spot Vasodilation
• Wheal Leakage of vessels
• Flare Axon reflex. Stimulation of sensory afferents signals itchy pain. Antidromic impulses from
branches of the axon cause release of chemicals causing nearby flare.
Histamine receptors
• 3 types (H1, H2 and H3)
• All are 7 transmembrane spanning G coupled receptors
• When histamine binds, it causes a conformational change which exposes a region on the intracellular tail of the
receptor which allows G proteins to bind.
Receptor subtype Location Prototype blocker Signalling molecule
H1 Smooth muscle Diphenhydramine PLC, IP3, DAG
Microvessels
Endothelium
Sensory nerves
H2 Stomach Cimetidine Increase in cAMP
Heart
Mast cells (autoinhibition)
Endothelium
(smooth muscle)
By Duy Thai, 1997 Pharmacology Semester 1 page 4 of 6
(lymphocytes)
H3 Nerves Impromidine G coupled
Brain Ca2+ entry
(gut)
(heart)
You must know the drugs listed on the handout
Signal transduction
• H1 receptor
• G protein
• Activates phospholipase C which causes release of IP3 and DAG
• IP3 stimulates release of intracellular Ca2+ stores
• DAG (in conjunction with Ca2+) activates protein kinase C
• Free intracellular Ca2+ may also activate Ca2+ dependent protein kinases
• e.g. myosin light chain kinase in smooth muscle (causing contraction)
• May also activate phospholipase A2 which causes production of eicosanoids
• H2 receptor
• G protein
• Activates adenylate cyclase
• Incrrease in cAMP
• H3 receptor
• G protein mediated Ca2+ entry from the extracellular compartment into the cell (note the difference to H1
receptors where the increase in cytosolic Ca2+ is from intracellular stores.)
Histamine in the brain
• Histamine is synthesised and degraded by enzymes in synaptosomes.
• H1 receptors in brain
• Found in hypothalamus
• Affect wakefulness (many antihistamines cause drowsiness)
• Appetite suppression
• H1/H2 receptors involved in:
• Drinking
• Thermoregulation
• Secretion of ADH
• Blood pressure regulation
• H3 receptors
• Poorly understood. Modulate H1 effects?
Histamine in the heart
• Increase in atrial and ventricular force via H2 receptors (increase in Ca2+)
• Increase in heart rate by reducing the diastolic depolarisation time is the SA node (H2 receptors)
• Decrease in the AV conduction time (H1 receptors)
• Arrhythmias (only in high doses)
• The above effects of histamine on the heart are only in experimental conditions. In the body, these effects do not
show any significance since they are overshadowed by the baroreflex, which is a more powerful controller of heart
rate and contractility.
Histamine in smooth muscles
• Bronchial smooth muscle H1: contraction is the dominant pathway
• Gut smooth muscle H2: relaxation (via increase in cAMP) is a weak minor compensation
(modulates H1 effects of constriction)
Histamine in the vessels
• H1 activates endothelial relaxation via NO (+PGI2)
By Duy Thai, 1997 Pharmacology Semester 1 page 5 of 6
• H2 activates a slower onset of direct relaxation. (some micorcirculations are very sensitive to histamine, so that a
H1 spasm may dominate)
Histmaine on the microvasculature
• Contrary to popular belief, histamine does NOT increase capillary permeability.
• Histamine works on the post capillary venule to increase the gap formation between endothelial cells, causing
plasma leakage. (Especially in acute inflammation)
• Histamine may increase the gap formation in 2 possible ways:
• Active contraction of the actin filaments in the endothelial cells.
• Transient de-adhesion
• The endothelial cells are attached to each other via tight junctions. There are also tiny elastic
filaments which are tonically active and will tend to pull the cells apart if there is no physical
connection between the cells. Histamine may cause a loss of adhesion of the tight junctions,
allowing the elastic filaments to cause tiny openings between the endothelial cells.
Agonists and antagonists
• Agonists
• No therapeutic use
• Only used in specialties such as in bronchial hypereactivity testing for asthmatics
• Used by dermatologists as a positive control to test for allergen reactivity.
• Antagonists
• H1 antagonists are used to treat mild allergies
• Hay fever
• Allergic conjunctavitis
• Rhinitis
• Some skin disorders
• Other “potential” uses of H1 antagonists
• Colds (useless, may even be harmful due to their sedative effect)]
• Asthma (never used any more, even though the airways of the asthmatic are filled with mast
cells)
• Useful in histamine overproduction diseases
• Motion sickness (not as effective as ascopolamine)
• Vomiting (not as good as ondansetron)
• H2 antagonists are often used to treat:
• Peptic ulcers
• Dyspepsia/heart burn
• Gastric reflux
Classic H1 antagonists
• Are seadtive
• Often short acting (3 – 6 hours)
• May have significant anti-cholinergic action
• See the handout for the names of the drugs (need to know!)
Non sedating (new generation) H1 antagonists
• Low or zero CNS penetration
• Long acting
• Little anticholinergic effects
• See the handout for the names of the drugs (need to know!)
• An important points about some of the new generation drugs:
• Polymorphic Ventricular Tachycardia (PVT) may occur with astemizole and terfenadine if
these drugs are taken in high doses or in conjunction with certain macrolide antibiotics
(erythromycin and clarithromycin) and/or certain anti-fungal drugs (ketaconazole and
By Duy Thai, 1997 Pharmacology Semester 1 page 6 of 6
itraconazole). These other drugs inhibit a hepatic cytochrome p450 enzyme which is required
for the metabolism of these 2 H1 antagonists.
H2 antagonists
• Also on the handout, but I will put them here anyway since there are not many
• Cimetidine
• Ranitidine
• Famotidine
• Nizatidine
How H2 receptors mediate acid secretion
Gastrin
G Parietal cell
Cl-
K+
ECL cell Histamine H2
↑ cAMP
H+
K+
M
Ach from Vagus
Proton pump
What we are expected to know
• H1 involved in allergy
• H2 involved in acid
• H3 involved in alertness
• Know:
• Synthesis and degradation pathway of histamine
• The distribution of histamine and mast cells
• Effects of histmine in humans
• Biology of mast cell degranulation
• Histamine receptor subtypes
• G proteins
• Transduction mechanisms
• Biology of acid secretion
• Uses of H1 blockers in allergy
• Differences between classical antagonists and the new generation
• Why H2 blockers are used to treat ulcers
• Be able to give the names of the drugs in the handout
• The basis of PVT
You might also like
- Paper Trade TJS Elite v8.9.2 - S-O REVISEDDocument1,170 pagesPaper Trade TJS Elite v8.9.2 - S-O REVISEDBryan Dawi42% (12)
- 9autacoids 14010v9023022 Phpapp02Document109 pages9autacoids 14010v9023022 Phpapp02h3cn1r3100% (1)
- IRDAI Memorandum Jeevan Saral Final DraftDocument4 pagesIRDAI Memorandum Jeevan Saral Final DraftMoneylife FoundationNo ratings yet
- Histamine and AntihistaminesDocument26 pagesHistamine and Antihistaminesgloria anyebeNo ratings yet
- Autacoids: Group No. 1Document92 pagesAutacoids: Group No. 1Rohan Pal100% (1)
- Histamine and AntihistaminesDocument26 pagesHistamine and Antihistaminesgloria anyebeNo ratings yet
- Autacoids Final PDFDocument22 pagesAutacoids Final PDFIqra ShaikhNo ratings yet
- Antimicrobial Agents-NrsDocument24 pagesAntimicrobial Agents-NrsSusmita BeheraNo ratings yet
- Autacoids A4 56Document2 pagesAutacoids A4 56ahmedsalah565vvvNo ratings yet
- AutacoidsDocument39 pagesAutacoidszeyadthemedicNo ratings yet
- Histamine and Serotonin: by Srota Dawn. M - Pharma (Pharmacology)Document34 pagesHistamine and Serotonin: by Srota Dawn. M - Pharma (Pharmacology)Rosel SorianoNo ratings yet
- Histamine Ans Serotonin and Their Antagonists 2020 - 2021Document109 pagesHistamine Ans Serotonin and Their Antagonists 2020 - 2021Sil Ana Salissou AbdouNo ratings yet
- Autacoids For Med.Document140 pagesAutacoids For Med.Feysal AhmedNo ratings yet
- 10 - AutacoidsDocument67 pages10 - AutacoidscchatrumaNo ratings yet
- Histamine: Dr. Anil Kumar SaxenaDocument17 pagesHistamine: Dr. Anil Kumar SaxenaLuqman Al-Bashir FauziNo ratings yet
- Autocoids, Histamine and Antihistaminic-Dr - Jibachha Sah, M.V.SC, LecturerDocument27 pagesAutocoids, Histamine and Antihistaminic-Dr - Jibachha Sah, M.V.SC, Lecturerjibachha sahNo ratings yet
- Histamine ..Document30 pagesHistamine ..Sunanda mohanNo ratings yet
- Pheromones, AutacoidsDocument64 pagesPheromones, Autacoidskelvinmaina9993No ratings yet
- AutacoidsDocument43 pagesAutacoidsOdiete EfeNo ratings yet
- Local Media1236679140 PDFDocument12 pagesLocal Media1236679140 PDFNusrat JahanNo ratings yet
- Autacoid Lec 1 & 2Document45 pagesAutacoid Lec 1 & 2s.nilormee1201No ratings yet
- Clinical Science SessionDocument12 pagesClinical Science SessionSelvi Puspa SariNo ratings yet
- AntiAllergy and Immunosupresive DrugDocument51 pagesAntiAllergy and Immunosupresive Drugfachrie saputraNo ratings yet
- Farmakologi Antihistamin-RDY 2019Document50 pagesFarmakologi Antihistamin-RDY 2019fandi mnNo ratings yet
- Anti His Tami NicDocument24 pagesAnti His Tami Nicgoharianali3000No ratings yet
- Antihistamine Presentation-KhallDocument34 pagesAntihistamine Presentation-Khallmaxwell amponsahNo ratings yet
- AUTOCOIDS - HISTAMINESDocument7 pagesAUTOCOIDS - HISTAMINESneonoelsmkNo ratings yet
- Autacoids (Local Hormones) and Their Pharmacolo-Gical ModulationDocument75 pagesAutacoids (Local Hormones) and Their Pharmacolo-Gical ModulationAgung PutraNo ratings yet
- Autacoids Related DrugsDocument74 pagesAutacoids Related DrugsVikash KushwahaNo ratings yet
- HISTAMINEDocument29 pagesHISTAMINEShehu Baba AbdullahiNo ratings yet
- AutacoidsDocument124 pagesAutacoidsMuhammad Modu BulamaNo ratings yet
- Histamine and Anti HistaminesDocument12 pagesHistamine and Anti HistaminesshrikantNo ratings yet
- AntihistaminDocument46 pagesAntihistaminVenerandaNo ratings yet
- HistamineDocument11 pagesHistamineOoi Ah GuanNo ratings yet
- Bp503t Pcol Unit-IIIDocument38 pagesBp503t Pcol Unit-IIIAakkkNo ratings yet
- Histamine & Antihistaminics: DR - Vijay BhushanamDocument24 pagesHistamine & Antihistaminics: DR - Vijay BhushanamTazrian FarahNo ratings yet
- 4 AutacoidsDocument9 pages4 AutacoidsAli EllaffiNo ratings yet
- AUTACOIDSDocument7 pagesAUTACOIDSElijah ChiumyaNo ratings yet
- DR Tehniat ZahraDocument19 pagesDR Tehniat ZahraRphTehniat ZahraNo ratings yet
- Pharma 24 To 31Document233 pagesPharma 24 To 31Loai Mohammed IssaNo ratings yet
- Antihistamine Presentation KhallDocument34 pagesAntihistamine Presentation KhallAdam Aulia RahmanNo ratings yet
- AntihistamineDocument2 pagesAntihistamineMichelle CasilangNo ratings yet
- AutacoidsDocument14 pagesAutacoidsMahmoud AboudNo ratings yet
- Histamine Pharmacology 2024Document24 pagesHistamine Pharmacology 2024Sylvester AsareNo ratings yet
- Slides AUTACOIDSDocument29 pagesSlides AUTACOIDSStrange eeNo ratings yet
- Autacoids: AntagonistsDocument37 pagesAutacoids: AntagonistsJayaNo ratings yet
- AntihistaminDocument46 pagesAntihistaminVenerandaNo ratings yet
- Histamine and Antihistamines LatestDocument28 pagesHistamine and Antihistamines LatestAjay KumarNo ratings yet
- HistamineDocument16 pagesHistamineسویرا منیرNo ratings yet
- Week 12 - 13 LongDocument62 pagesWeek 12 - 13 LongTom perryNo ratings yet
- Katzung - AutacoidsDocument23 pagesKatzung - AutacoidsKhairul AimanNo ratings yet
- With Important Actions On Smooth MuscleDocument90 pagesWith Important Actions On Smooth MuscleGeraldine Marie Salvo100% (1)
- Presentation 1Document25 pagesPresentation 1christinaNo ratings yet
- HistamineDocument14 pagesHistamineArslan RajpootNo ratings yet
- 1 Inflammation - Allergy - 230522 - 113129Document58 pages1 Inflammation - Allergy - 230522 - 113129Csparkz beautyNo ratings yet
- Kimia Farmasi: AntihistaminDocument25 pagesKimia Farmasi: AntihistaminchristinaNo ratings yet
- Autocoids 2Document68 pagesAutocoids 2Maryam NisaNo ratings yet
- Lipoproteins: - Function: Transport of Fat Soluble Substances - Types: 1) Chylomicron 2) VLDL 3) LDL 4) HDLDocument31 pagesLipoproteins: - Function: Transport of Fat Soluble Substances - Types: 1) Chylomicron 2) VLDL 3) LDL 4) HDLRexel EnnyNo ratings yet
- AntihistaminDocument44 pagesAntihistaminDWI RAHMA HALIDANo ratings yet
- Low Histamine Cooking in Your Instant Pot: 75 Easy Meals for Everyday HealingFrom EverandLow Histamine Cooking in Your Instant Pot: 75 Easy Meals for Everyday HealingNo ratings yet
- Pharm 3 IDocument3 pagesPharm 3 Iravi2like100% (1)
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 4 CDocument4 pagesPharm 4 Cravi2likeNo ratings yet
- Pharm 4 ADocument3 pagesPharm 4 Aravi2likeNo ratings yet
- Pharm 3 BDocument3 pagesPharm 3 Bravi2likeNo ratings yet
- By Duy Thai, 1997: Choline AcetyltransferaseDocument4 pagesBy Duy Thai, 1997: Choline Acetyltransferaseravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 3 eDocument3 pagesPharm 3 eravi2likeNo ratings yet
- Pharm 3 CDocument3 pagesPharm 3 Cravi2likeNo ratings yet
- By Duy Thai, 1997: Diffuse Out of Nerve Terminal and Act On COMPT in External TissuesDocument5 pagesBy Duy Thai, 1997: Diffuse Out of Nerve Terminal and Act On COMPT in External Tissuesravi2likeNo ratings yet
- Anti Inflammatory Drugs, by Duy Thai, 1997Document4 pagesAnti Inflammatory Drugs, by Duy Thai, 1997ghinsavitNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2like100% (2)
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Pharm 1 HDocument6 pagesPharm 1 Hravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 5Document5 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 5ravi2likeNo ratings yet
- Stimulates Ca ") .: 1997 Pharmacology Semester 1 Page 1 of 6Document6 pagesStimulates Ca ") .: 1997 Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- 1997 Pharmacology Semester 1 Page 1 of 6Document6 pages1997 Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- E Valency Ion Ion: A - Ve Value Means A Net Flow Into The CellDocument5 pagesE Valency Ion Ion: A - Ve Value Means A Net Flow Into The Cellravi2likeNo ratings yet
- Aspirin Irreversibly Inhibits Cyclo-Oxygenase: Pharmacology Semester 1 Page 1 of 5Document6 pagesAspirin Irreversibly Inhibits Cyclo-Oxygenase: Pharmacology Semester 1 Page 1 of 5ravi2likeNo ratings yet
- Pharm 1 GDocument3 pagesPharm 1 Gravi2likeNo ratings yet
- Pharm 1 eDocument3 pagesPharm 1 eravi2likeNo ratings yet
- Presence of An Antagonist.: Pharmacology Semester 1 Page 1 of 5Document5 pagesPresence of An Antagonist.: Pharmacology Semester 1 Page 1 of 5ravi2like100% (1)
- 1997 Pharmacology Semester 1 Page 1 of 4Document4 pages1997 Pharmacology Semester 1 Page 1 of 4ravi2like100% (1)
- Pharm 1 ADocument3 pagesPharm 1 Aravi2likeNo ratings yet
- Kelim Rate of Drug Elimination From The Plasma T Time: Pharmacology Semester 1 Page 1 of 6Document6 pagesKelim Rate of Drug Elimination From The Plasma T Time: Pharmacology Semester 1 Page 1 of 6ravi2likeNo ratings yet
- 1997 Pharmacology Semester 1 Page 1 of 7Document7 pages1997 Pharmacology Semester 1 Page 1 of 7ravi2likeNo ratings yet
- Time Value of Money: Compound InterestDocument28 pagesTime Value of Money: Compound InterestkateNo ratings yet
- Cad Notes PDFDocument61 pagesCad Notes PDFSuresh Natarajan100% (3)
- Bus FestoDocument82 pagesBus FestoraphvalonNo ratings yet
- Writing Research Papers James D Lester PDFDocument4 pagesWriting Research Papers James D Lester PDFgahebak1mez2100% (1)
- Inv - 1124003027 - Po - 407097662 - VR - 407097662 - (Top Synthetic Rubber)Document1 pageInv - 1124003027 - Po - 407097662 - VR - 407097662 - (Top Synthetic Rubber)shamirah98No ratings yet
- Applied Sciences: Determination of PH in Powdered Concrete Samples or in SuspensionDocument10 pagesApplied Sciences: Determination of PH in Powdered Concrete Samples or in SuspensionKarthick MNo ratings yet
- Bar Charts in ResearchDocument5 pagesBar Charts in ResearchPraise NehumambiNo ratings yet
- New MONDDocument7 pagesNew MONDVernonNo ratings yet
- WITH SOLUTIONS Pipe Connection and Three ReservoirDocument46 pagesWITH SOLUTIONS Pipe Connection and Three ReservoirMark PulongbaritNo ratings yet
- Lec 3Document32 pagesLec 3wjeelaniNo ratings yet
- B.Sc. SEM IDocument4 pagesB.Sc. SEM IsameerNo ratings yet
- Ghirri - Redutores - Classificação - Um-Vs-Rev.20.06.06-Eng3Document35 pagesGhirri - Redutores - Classificação - Um-Vs-Rev.20.06.06-Eng3Jeferson DantasNo ratings yet
- ScrewCompressors XRV204 SpecSheet DigitalDocument1 pageScrewCompressors XRV204 SpecSheet DigitalBarros Arias EnriqueNo ratings yet
- Home Learning IB GR 12 June 4, 7 - 11Document6 pagesHome Learning IB GR 12 June 4, 7 - 11Arnel Ordas AvilaNo ratings yet
- Phrasal Verb LESSON FINAL 1701874327225Document67 pagesPhrasal Verb LESSON FINAL 1701874327225bgt dfgNo ratings yet
- LPP - orDocument12 pagesLPP - orbharat_v79No ratings yet
- LLM Placement Brochure 2021 22 V15Document24 pagesLLM Placement Brochure 2021 22 V15Bala KumaranNo ratings yet
- Sarah Tchoukaleff ResumeDocument1 pageSarah Tchoukaleff Resumeapi-261081956No ratings yet
- Ad 345Document1 pageAd 345Vojtech ChalupaNo ratings yet
- 2008 Bullentin Rel. 2-DataDocument5 pages2008 Bullentin Rel. 2-DataKalai SelvanNo ratings yet
- Valuecent Profile PDFDocument9 pagesValuecent Profile PDFAP Advisory BVNo ratings yet
- Individual Taxpayers Tax Filing ExercisesDocument3 pagesIndividual Taxpayers Tax Filing ExercisesKIM RAGANo ratings yet
- Workout ChartDocument1 pageWorkout ChartAsadullah JawaidNo ratings yet
- BELOW KNEE AMPUTATION EditDocument16 pagesBELOW KNEE AMPUTATION EditPutri AyuNo ratings yet
- 2.1 Theory of Metal Cutting Q&A For StudentDocument8 pages2.1 Theory of Metal Cutting Q&A For Studentnikhilbatham0% (1)
- TIFDDocument1 pageTIFDAtulNo ratings yet
- Rural Development: 14.1. Rural Scenario in Tamil NaduDocument17 pagesRural Development: 14.1. Rural Scenario in Tamil NaduhinduNo ratings yet
- 8 SWOT Analysis Tools For Small Businesses: 1. SmartsheetDocument3 pages8 SWOT Analysis Tools For Small Businesses: 1. SmartsheetSantu BiswaaNo ratings yet