Professional Documents
Culture Documents
Y 430 A
Y 430 A
Uploaded by
LuisaGordonOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Y 430 A
Y 430 A
Uploaded by
LuisaGordonCopyright:
Available Formats
Lyofast Y 430 A
Description Lyofast Y 430 A consists of specifically selected strains of Streptococcus
thermophilus producing EPS and low content of Lactobacillus delbrueckii ssp.
bulgaricus.
Lyofast Y 430 A ensures a uniform and controlled production of very mild set and
stirred yoghurt with high viscosity.
Application Sprinkle the culture powder directly into process milk under aseptic conditions ensuring
that the culture is well dispersed by gentle stirring. The following may be used as
inoculation guidelines:
Product UC/100 l Product UC/100 l
Yoghurt, short set 2.0-4.0 Yoghurt, long set 0.5-1.0
Rotation The recommended rotation is Lyofast Y 432 A.
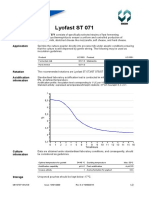
Acidification Standardised laboratory acidification test is conducted in milk powder, reconstituted at
information 9%, at defined temperature.
Acidification profile: inoculation level corresponding to 1 UC per 100 litres milk.
Standard activity: expressed as temperature/time/pH relations: 43°C/7 hours/pH 4.6 ± 0.1.
Culture Data are obtained under standardised laboratory conditions, and consequently, should
information be considered as guidelines:
Optimal temperature for growth 43°C Aroma formation for yoghurt +
Acidification capability pH 4.0 Texture formation 6±1.5 sec/g
Post-acidification ∆ pH 0.24
Storage Unopened pouches should be kept below -17°C.
M91Y430A/6/UK/0 Issue: 19/01/2009 Rev 6 of 24/11/2016 1/2
Lyofast Y 430 A
Package data The freeze-dried culture is packed in waterproof and airproof aluminium pouches. The
packaging material is food grade.
Shelf life 18 months when stored below -17°C.
Heavy metal Pb (lead) < 1 ppm
specification Hg (mercury) < 0.03 ppm
Cd (cadmium) < 0.1 ppm
* Analysed on regular basis.
Microbiological Bacillus cereus <100 CFU/g Method: Sacco M10 (1)
specification Coagulase positive staphylococci* <10 CFU/g Method: Sacco M11(2)
Enterobacteriaceae <10 CFU/g Method: Sacco M02 (3)
Escherichia coli <1 CFU/g Method: Sacco M27 (4)
Listeria monocytogenes* Not detected in 25 g Method: Sacco M13 (5)
Moulds & yeasts <10 CFU/g Method: Sacco M03 (6)
Salmonella spp.* Not detected in 25 g Method: Sacco M12 (7)
* Analysed on regular basis. All analytical methods are available upon request.
(1)ISO 7932; (2)ISO 6888-1-2; (3)ISO 21528-1-2; (4)ISO11866-1-2/IDF 170-1-2; (5)ISO 11290-1-2; (6)ISO
6611/IDF 94; (7)ISO 6785/IDF 93.
GMO The microbial strains are not genetically modified (GMO) in accordance with the
Directive 2001/18/EC. The strains are isolated from natural sources. The raw materials
used are also GMO free in accordance with Regulation (EC) No. 1829/2003 and
Regulation (EC) No. 1830/2003.
Allergens The raw materials used are generally based on dairy ingredients. All materials are free
of the following components and their derivatives: peanuts, nuts, sesame seeds, eggs,
fish, shellfish, molluscs, crustaceans, cereals containing gluten, celery, mustard,
soybeans, lupin, sulphur dioxide and sulphites.
Safety information Material Safety Data Sheet available on www.saccosrl.it
Certificate Lot certificate available upon request.
Certifications Sacco S.r.l. is UNI EN ISO 9001:2008 certified since 1998, ISO 22000:2005 and FSSC
22000 certified since 2014. Sacco cultures are generally Kosher and Halal approved
except for surface ripening cultures.
Service Please contact your distributor for guidance and instructions for your choice of culture
and processing. Information about additional package sizes and sales units is also
available upon request.
Liability This information is based on our knowledge trustworthy and presented in good faith. No
guarantee against patent infringement is implied or inferred.
M91Y430A/6/UK/0 Issue: 19/01/2009 Rev 6 of 24/11/2016 2/2
You might also like
- Beneopro VWG 75 Food: Product SheetDocument3 pagesBeneopro VWG 75 Food: Product SheetJosé RoqueNo ratings yet
- AEI-Byrne Biotechnology Protest Industry 2003Document17 pagesAEI-Byrne Biotechnology Protest Industry 2003Jay ByrneNo ratings yet
- Gmo WorksheetDocument2 pagesGmo Worksheetscribd100% (1)
- TC Lyofast-Y 456-B EN 230616Document2 pagesTC Lyofast-Y 456-B EN 230616Anel MamaniNo ratings yet
- Fish and ChipsDocument2 pagesFish and Chipsyrynukaaa0% (1)
- W.D. BigelowDocument10 pagesW.D. BigelowCarrie RodriquezNo ratings yet
- Thermophysical Properties of Orange JuiceDocument14 pagesThermophysical Properties of Orange JuiceGladys González González100% (1)
- Efecto Del Presecado Sobre La Cinética de Pérdida de Humedad y Absorción de Aceite Durante La Fritura de Grasa ProfundaDocument8 pagesEfecto Del Presecado Sobre La Cinética de Pérdida de Humedad y Absorción de Aceite Durante La Fritura de Grasa Profundafaty hdezNo ratings yet
- TC Lyofast-Y-438Document2 pagesTC Lyofast-Y-438Marck Anderson Mamani AvilesNo ratings yet
- Lyofast MTX 432 ENDocument2 pagesLyofast MTX 432 ENLuisaGordonNo ratings yet
- LyofastST071 TechDocument2 pagesLyofastST071 Techchrist castroNo ratings yet
- Lyofast MO 242: DescriptionDocument2 pagesLyofast MO 242: DescriptionMilena CastroNo ratings yet
- Ficha Tecnica Cultivo para QuesoDocument2 pagesFicha Tecnica Cultivo para QuesoLeyarope RoRoNo ratings yet
- Lyofast MW 039 SDocument2 pagesLyofast MW 039 SNilo C Cervantes ChipaNo ratings yet
- Lyofast ST 081Document2 pagesLyofast ST 081jimeNo ratings yet
- 21 07 22 Y456bDocument3 pages21 07 22 Y456bYogures ZhuaNo ratings yet
- Lyofast LR B: DescriptionDocument2 pagesLyofast LR B: DescriptionjimeNo ratings yet
- Ficha Tecnica Sab 440ADocument0 pagesFicha Tecnica Sab 440AAlexander GuzmanNo ratings yet
- LayofastDocument2 pagesLayofastMuhammad Usman AkramNo ratings yet
- Sacco 450b Grafico PH Vs TDocument2 pagesSacco 450b Grafico PH Vs TLaura Vanessa AriasNo ratings yet
- VALIREN BF 300 v2Document2 pagesVALIREN BF 300 v2info.salvecesarNo ratings yet
- Product Specifications:Rokiškio Sūris Cheese 45% Fat.i D.M.Document2 pagesProduct Specifications:Rokiškio Sūris Cheese 45% Fat.i D.M.Jason ThorntonNo ratings yet
- BM099 Agar+Glucoza FioleDocument3 pagesBM099 Agar+Glucoza FioleKeep CalmNo ratings yet
- Lacprodan Clear Shake P1279Document2 pagesLacprodan Clear Shake P1279Alexandre FarrantNo ratings yet
- Rosemary LeavesDocument3 pagesRosemary LeavesmarouaNo ratings yet
- Biolife: Nutrient AgarDocument2 pagesBiolife: Nutrient AgarZoza SalamaNo ratings yet
- BK185Document3 pagesBK185Keep CalmNo ratings yet
- Product SpecificationsDocument1 pageProduct SpecificationsMichael LandryNo ratings yet
- Merck Rebrand - 100908 - 1907Document5 pagesMerck Rebrand - 100908 - 1907Paula BautistaNo ratings yet
- Specification SugarDocument4 pagesSpecification SugarAlvin Yoga FahrurroziNo ratings yet
- f002441 White Truffle Nat SP en NewDocument2 pagesf002441 White Truffle Nat SP en Newgoran.vuckovic78No ratings yet
- Product Specifications: Montecampo CheeseDocument2 pagesProduct Specifications: Montecampo CheeseJason ThorntonNo ratings yet
- EAS 22 2006 Butter SpecificationDocument8 pagesEAS 22 2006 Butter SpecificationFelix MwandukaNo ratings yet
- Technical Sheet Specification-Organic-Chaga-ExtractDocument3 pagesTechnical Sheet Specification-Organic-Chaga-ExtractDana JuarezNo ratings yet
- Biokar Hal 1Document4 pagesBiokar Hal 1yehezgiankaNo ratings yet
- BK115 133 BM013 v7Document5 pagesBK115 133 BM013 v7Keep CalmNo ratings yet
- Mrs Agar: Reference: Product: Scharlau Microbiology - Technical Data SheetDocument2 pagesMrs Agar: Reference: Product: Scharlau Microbiology - Technical Data SheetOlusegun OlasugbaNo ratings yet
- Bo-Ps - Hsi-A4-15-007 01-05-2021Document4 pagesBo-Ps - Hsi-A4-15-007 01-05-2021Hakan ÇİÇEKNo ratings yet
- Arginineglucose Yeast Extract Agar - Technicaldetails - 9720210526.050253Document3 pagesArginineglucose Yeast Extract Agar - Technicaldetails - 9720210526.050253MSc Penélope MeloNo ratings yet
- Parsley Leaf - All Formats Oht Spec 007 Rev2Document5 pagesParsley Leaf - All Formats Oht Spec 007 Rev2amiraabdelnby310No ratings yet
- 1301 en 2Document2 pages1301 en 2lgoNo ratings yet
- Steiktur Laukur 2 5 KGDocument6 pagesSteiktur Laukur 2 5 KGOmer MukhtarNo ratings yet
- Specification - FactoryDocument3 pagesSpecification - Factorythomas.rohanNo ratings yet
- Ifu BpaDocument4 pagesIfu BpaoktaNo ratings yet
- Uganda Notification On Powdered Silver Cyprinid - Nairobi - Uganda - 07!25!2021Document9 pagesUganda Notification On Powdered Silver Cyprinid - Nairobi - Uganda - 07!25!2021MajugoNo ratings yet
- Lactina Starter Cultures Yogurt enDocument2 pagesLactina Starter Cultures Yogurt enDaniel MwangiNo ratings yet
- 156-Skimmed Milk Medium Heat - Rev 1a - GBDocument2 pages156-Skimmed Milk Medium Heat - Rev 1a - GBMostafa AlakhliNo ratings yet
- Product Specifications: Edam CheeseDocument1 pageProduct Specifications: Edam CheeseJason ThorntonNo ratings yet
- Buffered Charcoal Yeast Extract Agar Technicaldetails 27020230327.100923Document2 pagesBuffered Charcoal Yeast Extract Agar Technicaldetails 27020230327.100923ikinNo ratings yet
- RIG Vital Wheat Gluten PS2013 00 V200115 01Document2 pagesRIG Vital Wheat Gluten PS2013 00 V200115 01ahetNo ratings yet
- 8 Crude Sunflower Oil en LQS SBZ21DDocument3 pages8 Crude Sunflower Oil en LQS SBZ21Dalexandermemphis1No ratings yet
- SNI 01-2332.2-2006 Salmonella - Id.enDocument29 pagesSNI 01-2332.2-2006 Salmonella - Id.enEka SaputraNo ratings yet
- Microbial Detection - Ariake Europe IndonesiaDocument14 pagesMicrobial Detection - Ariake Europe Indonesiaannisa nur ainiNo ratings yet
- Bacillus Cereus Selective Agar Base (MYP) ISO: Industry RegulationsDocument2 pagesBacillus Cereus Selective Agar Base (MYP) ISO: Industry RegulationsLong ManNo ratings yet
- MM Rebrand 107993 - 1202 - 2Document4 pagesMM Rebrand 107993 - 1202 - 2Wahyu NugrahaNo ratings yet
- ISP5 (Glycerol Asparagine Medium)Document2 pagesISP5 (Glycerol Asparagine Medium)zemouraNo ratings yet
- Violet Red Bile Agar VDocument2 pagesViolet Red Bile Agar Vmustea_ana9616No ratings yet
- SPEC-34011 - Specification Meatless Dehydrated Faba Flake Coarse DFF - 11Document3 pagesSPEC-34011 - Specification Meatless Dehydrated Faba Flake Coarse DFF - 11n.christingerNo ratings yet
- CP21CL - Cengkeh Cloves Ground v9Document5 pagesCP21CL - Cengkeh Cloves Ground v9ptsathrusi kiNo ratings yet
- ALASKO - IQF Wild Blueberries (Grade A) - FTDocument3 pagesALASKO - IQF Wild Blueberries (Grade A) - FTCalidad - IndufrutNo ratings yet
- Haccp Case Study JamDocument11 pagesHaccp Case Study Jamdjureziii100% (2)
- Official - ncm1004 - Harlequin Listeria Chromogenic Agar According To Ottaviani and Agosti - Technical Specifications - en UsDocument3 pagesOfficial - ncm1004 - Harlequin Listeria Chromogenic Agar According To Ottaviani and Agosti - Technical Specifications - en UsJessica ChavezNo ratings yet
- Lyofast MTX 432 ENDocument2 pagesLyofast MTX 432 ENLuisaGordonNo ratings yet
- SecadoDocument5 pagesSecadoLuisaGordonNo ratings yet
- AquaLab Series FourDocument121 pagesAquaLab Series FourLuisaGordonNo ratings yet
- Acrylamide Formation in Plantain (Musa Paradisiaca) ChipsDocument8 pagesAcrylamide Formation in Plantain (Musa Paradisiaca) ChipsLuisaGordonNo ratings yet
- 0-1Document15 pages0-1Hồng PhúcNo ratings yet
- Environment The Science Behind The Stories Canadian 3rd Edition Whitgott Test BankDocument26 pagesEnvironment The Science Behind The Stories Canadian 3rd Edition Whitgott Test Bankfreyahypatias7j100% (33)
- STS Research FinalllDocument35 pagesSTS Research FinalllAngelica Faye DuroNo ratings yet
- Inheritance 1 3 3Document22 pagesInheritance 1 3 3eeveesylvionumbriounNo ratings yet
- Price List Jun23 AgrocooperDocument2 pagesPrice List Jun23 Agrocooper4Ever Comissária de DespachosNo ratings yet
- GMOs - 522 Document 9-24-13Document2 pagesGMOs - 522 Document 9-24-13GMOFreeWashingtonNo ratings yet
- GM FoodsDocument7 pagesGM Foodsapi-276860380No ratings yet
- Pew Science and Society ReportDocument111 pagesPew Science and Society ReportcraignewmanNo ratings yet
- Speak About Blurring The Line Between Art and Science. What Do You Think of The Future of Art?Document8 pagesSpeak About Blurring The Line Between Art and Science. What Do You Think of The Future of Art?Анастасия КривобокNo ratings yet
- Examen Final Regular Inglés I (NOTA 10) - Colombo - Agustina-Ing. - en - Alimentos-173479-11-02-21Document5 pagesExamen Final Regular Inglés I (NOTA 10) - Colombo - Agustina-Ing. - en - Alimentos-173479-11-02-21Freak GamerGtNo ratings yet
- Reflective Essay Cri D ScienceDocument7 pagesReflective Essay Cri D Scienceapi-462907176No ratings yet
- Path Modeling The Antecedent Factors (Spais and Vasileiou)Document28 pagesPath Modeling The Antecedent Factors (Spais and Vasileiou)silmiNo ratings yet
- Genetically Modified Foods SlidesDocument18 pagesGenetically Modified Foods SlidesBicycle ThiefNo ratings yet
- Reading WEEK 6Document30 pagesReading WEEK 6Truong Man KhueNo ratings yet
- ScriptDocument2 pagesScriptKenneth FloresNo ratings yet
- XIII Toxicological Evaluation of Proteins Introduced Into Food CropsDocument33 pagesXIII Toxicological Evaluation of Proteins Introduced Into Food CropsRichard Enmanuel Quispe PacoriNo ratings yet
- Genetic Modification of CropsDocument13 pagesGenetic Modification of CropsYashraj DokaniaNo ratings yet
- Halal Awareness Webinar June 30 2020 PDFDocument47 pagesHalal Awareness Webinar June 30 2020 PDFMuhminHassanNo ratings yet
- GMO Certification SchemeDocument25 pagesGMO Certification Schemetheanjum1980No ratings yet
- Bahasa Inggris - Lily Anvionita - A1B121091Document3 pagesBahasa Inggris - Lily Anvionita - A1B121091Lily AnvionitaNo ratings yet
- 4th Biotechnology 8 TestDocument6 pages4th Biotechnology 8 TestXianNo ratings yet
- Thesis Statement Against GM FoodsDocument4 pagesThesis Statement Against GM Foodsjackiehintonwashington100% (2)
- Mun Position PaperDocument1 pageMun Position Paperapi-458726271No ratings yet
- CHAPTER VII Genetically Modified OrganismDocument16 pagesCHAPTER VII Genetically Modified OrganismMaeann FernandezNo ratings yet
- The Truths of Genetically Modified Foods EssayDocument4 pagesThe Truths of Genetically Modified Foods Essayapi-259536704No ratings yet
- Genetically Modified OrganismsDocument2 pagesGenetically Modified Organismsapi-323374257No ratings yet
- Technological Development of Agriculture and Its Great DilemmasDocument3 pagesTechnological Development of Agriculture and Its Great DilemmasAngelica Mae CornejoNo ratings yet
- IELTS Hammer - Writing Task 2Document20 pagesIELTS Hammer - Writing Task 2Samith Fernando100% (1)