Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
596 viewsClassification of Matters Worksheet 4 Answers
Classification of Matters Worksheet 4 Answers
Uploaded by
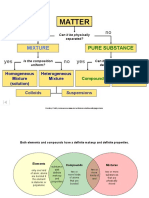
Mon Agulto LomedaThis document provides information about classifying different types of matter. It defines elements, compounds, homogeneous mixtures, and heterogeneous mixtures. A mixture contains two or more substances that retain their chemical properties, while a compound is a single substance with its own properties. Solutions are homogeneous mixtures where particles are evenly distributed at a microscopic level. Colloids have slightly larger insoluble particles, and suspensions have even larger particles that settle over time. The document also classifies properties and changes as either chemical or physical.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You might also like
- MYP - 1 - Unit - 2 - The - Properties - of - Matter - Part - 2Document43 pagesMYP - 1 - Unit - 2 - The - Properties - of - Matter - Part - 2vandana giriNo ratings yet
- 6th Grade-Post-Assessment Directions and Answer KeyDocument7 pages6th Grade-Post-Assessment Directions and Answer KeySULTAN SURGANo ratings yet
- Separation Techniques WorksheetDocument2 pagesSeparation Techniques WorksheetyuniNo ratings yet
- Basic Concepts About Matter: Test BankDocument12 pagesBasic Concepts About Matter: Test BankRalph Aubrey CulhiNo ratings yet
- Project Proposal MURAL PAINTINGDocument2 pagesProject Proposal MURAL PAINTINGMon Agulto LomedaNo ratings yet
- Atoms and Elements Worksheet 1 Year 1 ScienceDocument10 pagesAtoms and Elements Worksheet 1 Year 1 SciencemizterdeeNo ratings yet
- MYP Science 10: Lab Report Writing GuideDocument2 pagesMYP Science 10: Lab Report Writing GuideTiberiuNo ratings yet
- Efficiency WorksheetDocument2 pagesEfficiency Worksheetchpwalker50% (2)
- Naming Alkanes Worksheet 2Document2 pagesNaming Alkanes Worksheet 2Kamariah Ismail100% (1)
- Trends Graph WorksheetDocument6 pagesTrends Graph Worksheetmamazookeepr100% (4)
- Science6 1 Quarter Practice Test Fill in The Blanks:: DecantationDocument4 pagesScience6 1 Quarter Practice Test Fill in The Blanks:: DecantationPinky Laysa100% (1)
- Cycles of Matter WorksheetDocument3 pagesCycles of Matter WorksheetnaremanNo ratings yet
- Classification of MatterDocument30 pagesClassification of Mattershasagail100% (1)
- States of Matter Solid, Liquid, Gas, and Plasma. Solids LiquidsDocument6 pagesStates of Matter Solid, Liquid, Gas, and Plasma. Solids LiquidsWaleedSubhanNo ratings yet
- Bonding PowerpointDocument14 pagesBonding Powerpointᴍɪᴋᴋɪᴋᴀᴢᴇ100% (2)
- Separating Techniques WorksheetDocument2 pagesSeparating Techniques WorksheetPehel MehtaNo ratings yet
- Put Tick Mark For The Correct AnswerDocument4 pagesPut Tick Mark For The Correct AnswerVANSHIKA AGARWALNo ratings yet
- Separation TechniquesDocument19 pagesSeparation TechniquesLenin Prabhu100% (4)
- 8H The Rock Cycle Multiple Choice TestDocument3 pages8H The Rock Cycle Multiple Choice Testapi-3698146100% (2)
- Worksheet - Classification of Matter - KeyDocument1 pageWorksheet - Classification of Matter - KeyVaughnNo ratings yet
- 04 Worksheet 1 Separating MixturesDocument4 pages04 Worksheet 1 Separating MixturesSarahglen Ganob Lumanao100% (1)
- Form 2 8 Ionic BondingDocument64 pagesForm 2 8 Ionic BondingOsmany Madrigal100% (1)
- Physical and Chemical Change WorksheetDocument5 pagesPhysical and Chemical Change Worksheetmheojhun100% (1)
- Elements, Compounds, & MixturesDocument72 pagesElements, Compounds, & MixturesEvangelene Esquillo SanaNo ratings yet
- Separation Techniques Questions YR8Document5 pagesSeparation Techniques Questions YR8Bushra AkhtarNo ratings yet
- Separation Techniques WorksheetDocument2 pagesSeparation Techniques WorksheetJESSAH MAE GAMUTANNo ratings yet
- Ionic Bonding Part 1 EdexcelDocument4 pagesIonic Bonding Part 1 EdexcelKevin The Chemistry Tutor100% (1)
- Specialised CellsDocument9 pagesSpecialised CellsJanah Pauline AbunganNo ratings yet
- 02-04 Atomic Structure Worksheet - AnswersDocument2 pages02-04 Atomic Structure Worksheet - AnswersRSLNo ratings yet
- Animal and Plant CellDocument30 pagesAnimal and Plant CellNetty BontuyanNo ratings yet
- Biology Reviewer QuestionsDocument10 pagesBiology Reviewer QuestionsMICHELLE DE GUZMAN SOTTONo ratings yet
- Grade 7 Note On Separating TechniquesDocument8 pagesGrade 7 Note On Separating TechniquesBadass PolapainNo ratings yet
- Chemical Reactions and EquationsDocument8 pagesChemical Reactions and EquationsDog100% (1)
- GCSE Exam QuestionsDocument155 pagesGCSE Exam Questionsdanielphilip68No ratings yet
- Physical and Chemical Change WorksheetDocument1 pagePhysical and Chemical Change Worksheetapi-350245383No ratings yet
- Grade 6 Chemistry Annual Term Revision WorksheetsDocument9 pagesGrade 6 Chemistry Annual Term Revision WorksheetsAbhayNo ratings yet
- (GR) COMPLETE - Multiple Choice Quiz Questions - Chemistry PDFDocument30 pages(GR) COMPLETE - Multiple Choice Quiz Questions - Chemistry PDFYasir AkhunNo ratings yet
- 4 - Unicellular Structural AdaptationsDocument12 pages4 - Unicellular Structural AdaptationsNayani Snigdha0% (1)
- Chemistry Final Exam For Grade 9Document5 pagesChemistry Final Exam For Grade 9debbie teferaNo ratings yet
- 5) Percent CompositionDocument11 pages5) Percent CompositionRamona Esteves100% (1)
- States of Matter: Paper 1: Practice TestDocument4 pagesStates of Matter: Paper 1: Practice TestSadaqat UllahNo ratings yet
- Quizzes 2Document3 pagesQuizzes 2api-254428474No ratings yet
- Practice Worksheet of Chemical BondingDocument2 pagesPractice Worksheet of Chemical Bondingch khakanNo ratings yet
- Elements, Compounds and MixturesDocument71 pagesElements, Compounds and MixturesAkshitSanghavi100% (1)
- Form 1 Chapter 3 - Three States of MatterDocument11 pagesForm 1 Chapter 3 - Three States of MatterMohd Safwan Mohd IsaNo ratings yet
- Physical and Chemical ChangeDocument10 pagesPhysical and Chemical ChangeAdrian CadizNo ratings yet
- Worksheet 1 - Chemical BondingDocument4 pagesWorksheet 1 - Chemical BondingFahd KhanNo ratings yet
- Long Quiz About Asexual and SexualDocument1 pageLong Quiz About Asexual and SexualLynne Tuiza EndonNo ratings yet
- Specialised CellsDocument34 pagesSpecialised CellsShaikBasheerAhmedNizami100% (1)
- Chapter Test On Sound WavesDocument1 pageChapter Test On Sound WavesryanmanubagNo ratings yet
- Separation Techniques Worksheet Ms Tay-1Document2 pagesSeparation Techniques Worksheet Ms Tay-1Anthony BensonNo ratings yet
- Pure Substances Vs MixturesDocument25 pagesPure Substances Vs Mixturesmisterbrowner100% (7)
- Name of Atom Common Ionic ChargeDocument2 pagesName of Atom Common Ionic ChargeMichael Rey MendozaNo ratings yet
- Separation TechniqueDocument8 pagesSeparation TechniqueachoeyzNo ratings yet
- Final Exam Gen. Chem1Document3 pagesFinal Exam Gen. Chem1Joemar GagnaoNo ratings yet
- Plant Reproduction QuizDocument3 pagesPlant Reproduction QuizLKM100% (1)
- THE PERIODIC TABLE - Multiple Choice Review QuestionsDocument5 pagesTHE PERIODIC TABLE - Multiple Choice Review Questionssaga_1150% (4)
- 1.2 Mole ConceptDocument66 pages1.2 Mole Conceptnurain syuhadaNo ratings yet
- Separation HW BOOK PDFDocument9 pagesSeparation HW BOOK PDFAngela KocevskaNo ratings yet
- Most Essential Learning CompetenciesDocument8 pagesMost Essential Learning CompetenciesKaterina TagleNo ratings yet
- Classification of Matter Worksheet #4: P A - C MDocument2 pagesClassification of Matter Worksheet #4: P A - C Mkaruro JorudenuNo ratings yet
- School Safety Assessment Tool For The Pilot Study On Face-To-Face of Learning ModalityDocument5 pagesSchool Safety Assessment Tool For The Pilot Study On Face-To-Face of Learning ModalityMon Agulto LomedaNo ratings yet
- Table 29. Number of Functional Computers in The School by Funding Source, Sy 2019-2020 (As of June 30, 2021)Document2 pagesTable 29. Number of Functional Computers in The School by Funding Source, Sy 2019-2020 (As of June 30, 2021)Mon Agulto LomedaNo ratings yet
- Project Proposal BONDPAPER 1Document1 pageProject Proposal BONDPAPER 1Mon Agulto LomedaNo ratings yet
- Project Proposal MURAL PAINTINGDocument2 pagesProject Proposal MURAL PAINTINGMon Agulto LomedaNo ratings yet
- Virgen Delas Flores High SchoolDocument2 pagesVirgen Delas Flores High SchoolMon Agulto LomedaNo ratings yet
- Virgen Delas Flores High SchoolDocument1 pageVirgen Delas Flores High SchoolMon Agulto LomedaNo ratings yet
- Schools Division of Bulacan: Virgen Dela Flores High SchoolDocument12 pagesSchools Division of Bulacan: Virgen Dela Flores High SchoolMon Agulto LomedaNo ratings yet
- Self Learning MaterialDocument39 pagesSelf Learning MaterialMon Agulto LomedaNo ratings yet
- Grade 8 Resectioning - AS OF SEPT. 12 PMDocument35 pagesGrade 8 Resectioning - AS OF SEPT. 12 PMMon Agulto LomedaNo ratings yet
- Grade 9 Resectioning List of Students Per Section UpdatedDocument13 pagesGrade 9 Resectioning List of Students Per Section UpdatedMon Agulto LomedaNo ratings yet
- Science - Grade 8 Supplementary Learning Resources Quarter IV: Roles of Organisms First Edition 2020Document23 pagesScience - Grade 8 Supplementary Learning Resources Quarter IV: Roles of Organisms First Edition 2020Mon Agulto LomedaNo ratings yet
Classification of Matters Worksheet 4 Answers
Classification of Matters Worksheet 4 Answers
Uploaded by
Mon Agulto Lomeda0 ratings0% found this document useful (0 votes)
596 views1 pageThis document provides information about classifying different types of matter. It defines elements, compounds, homogeneous mixtures, and heterogeneous mixtures. A mixture contains two or more substances that retain their chemical properties, while a compound is a single substance with its own properties. Solutions are homogeneous mixtures where particles are evenly distributed at a microscopic level. Colloids have slightly larger insoluble particles, and suspensions have even larger particles that settle over time. The document also classifies properties and changes as either chemical or physical.
Original Description:
Original Title
Classification of Matters Worksheet 4 Answers.doc
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides information about classifying different types of matter. It defines elements, compounds, homogeneous mixtures, and heterogeneous mixtures. A mixture contains two or more substances that retain their chemical properties, while a compound is a single substance with its own properties. Solutions are homogeneous mixtures where particles are evenly distributed at a microscopic level. Colloids have slightly larger insoluble particles, and suspensions have even larger particles that settle over time. The document also classifies properties and changes as either chemical or physical.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
596 views1 pageClassification of Matters Worksheet 4 Answers
Classification of Matters Worksheet 4 Answers
Uploaded by
Mon Agulto LomedaThis document provides information about classifying different types of matter. It defines elements, compounds, homogeneous mixtures, and heterogeneous mixtures. A mixture contains two or more substances that retain their chemical properties, while a compound is a single substance with its own properties. Solutions are homogeneous mixtures where particles are evenly distributed at a microscopic level. Colloids have slightly larger insoluble particles, and suspensions have even larger particles that settle over time. The document also classifies properties and changes as either chemical or physical.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 1
Classification of Matter Worksheet #4
PART A – COMPOSITION OF MATTER
1. Classify the following as element, compound, heterogeneous mixture, or solution
(homogeneous mixture).
a. hydrogen gas Element.
b. orange juice Heterogeneous mixture.
c. air It depends. Within roughly the same altitude, homogeneous
mixture; at vastly different altitudes, heterogeneous mixture.
d. carbon dioxide (CO2) Compounds.
2. Compare and contrast a mixture and a compound. How are they alike? How are they
different? Use complete sentences.
Similarity: a mixture and a compound both are composed of entity that retains chemical
properties (molecules, ions or atoms).
Differences: a mixture is made up with two or more entities (molecules, ions or atoms).
The composition of the mixture could be uniform (homogeneous) or not uniform
(heterogeneous). A compound is composed of single entity (molecule, ions or atoms).
PART B – SOLUTIONS, COLLOIDS, AND SUSPENSIONS
3. For each type of mixture draw a picture representing the size and distribution of particles in
a liquid. (only the particles are drawn)
SOLUTION COLLOID SUSPENSION
PART C – PROPERTIES & CHANGES OF MATTER
4. Classify the following properties of matter as chemical (C) or physical (P).
a. flexible P (intensive) c. boils at 20C P (intensive)
b. combustible C d. low reactivity C
5. Classify the following as chemical (C) or physical (P) changes.
a. grapes fermenting C c. recycling aluminum P
b. copper melting P d. gasoline exploding C
You might also like
- MYP - 1 - Unit - 2 - The - Properties - of - Matter - Part - 2Document43 pagesMYP - 1 - Unit - 2 - The - Properties - of - Matter - Part - 2vandana giriNo ratings yet
- 6th Grade-Post-Assessment Directions and Answer KeyDocument7 pages6th Grade-Post-Assessment Directions and Answer KeySULTAN SURGANo ratings yet
- Separation Techniques WorksheetDocument2 pagesSeparation Techniques WorksheetyuniNo ratings yet
- Basic Concepts About Matter: Test BankDocument12 pagesBasic Concepts About Matter: Test BankRalph Aubrey CulhiNo ratings yet
- Project Proposal MURAL PAINTINGDocument2 pagesProject Proposal MURAL PAINTINGMon Agulto LomedaNo ratings yet
- Atoms and Elements Worksheet 1 Year 1 ScienceDocument10 pagesAtoms and Elements Worksheet 1 Year 1 SciencemizterdeeNo ratings yet
- MYP Science 10: Lab Report Writing GuideDocument2 pagesMYP Science 10: Lab Report Writing GuideTiberiuNo ratings yet
- Efficiency WorksheetDocument2 pagesEfficiency Worksheetchpwalker50% (2)
- Naming Alkanes Worksheet 2Document2 pagesNaming Alkanes Worksheet 2Kamariah Ismail100% (1)
- Trends Graph WorksheetDocument6 pagesTrends Graph Worksheetmamazookeepr100% (4)
- Science6 1 Quarter Practice Test Fill in The Blanks:: DecantationDocument4 pagesScience6 1 Quarter Practice Test Fill in The Blanks:: DecantationPinky Laysa100% (1)
- Cycles of Matter WorksheetDocument3 pagesCycles of Matter WorksheetnaremanNo ratings yet
- Classification of MatterDocument30 pagesClassification of Mattershasagail100% (1)
- States of Matter Solid, Liquid, Gas, and Plasma. Solids LiquidsDocument6 pagesStates of Matter Solid, Liquid, Gas, and Plasma. Solids LiquidsWaleedSubhanNo ratings yet
- Bonding PowerpointDocument14 pagesBonding Powerpointᴍɪᴋᴋɪᴋᴀᴢᴇ100% (2)
- Separating Techniques WorksheetDocument2 pagesSeparating Techniques WorksheetPehel MehtaNo ratings yet
- Put Tick Mark For The Correct AnswerDocument4 pagesPut Tick Mark For The Correct AnswerVANSHIKA AGARWALNo ratings yet
- Separation TechniquesDocument19 pagesSeparation TechniquesLenin Prabhu100% (4)
- 8H The Rock Cycle Multiple Choice TestDocument3 pages8H The Rock Cycle Multiple Choice Testapi-3698146100% (2)
- Worksheet - Classification of Matter - KeyDocument1 pageWorksheet - Classification of Matter - KeyVaughnNo ratings yet
- 04 Worksheet 1 Separating MixturesDocument4 pages04 Worksheet 1 Separating MixturesSarahglen Ganob Lumanao100% (1)
- Form 2 8 Ionic BondingDocument64 pagesForm 2 8 Ionic BondingOsmany Madrigal100% (1)
- Physical and Chemical Change WorksheetDocument5 pagesPhysical and Chemical Change Worksheetmheojhun100% (1)
- Elements, Compounds, & MixturesDocument72 pagesElements, Compounds, & MixturesEvangelene Esquillo SanaNo ratings yet
- Separation Techniques Questions YR8Document5 pagesSeparation Techniques Questions YR8Bushra AkhtarNo ratings yet
- Separation Techniques WorksheetDocument2 pagesSeparation Techniques WorksheetJESSAH MAE GAMUTANNo ratings yet
- Ionic Bonding Part 1 EdexcelDocument4 pagesIonic Bonding Part 1 EdexcelKevin The Chemistry Tutor100% (1)
- Specialised CellsDocument9 pagesSpecialised CellsJanah Pauline AbunganNo ratings yet
- 02-04 Atomic Structure Worksheet - AnswersDocument2 pages02-04 Atomic Structure Worksheet - AnswersRSLNo ratings yet
- Animal and Plant CellDocument30 pagesAnimal and Plant CellNetty BontuyanNo ratings yet
- Biology Reviewer QuestionsDocument10 pagesBiology Reviewer QuestionsMICHELLE DE GUZMAN SOTTONo ratings yet
- Grade 7 Note On Separating TechniquesDocument8 pagesGrade 7 Note On Separating TechniquesBadass PolapainNo ratings yet
- Chemical Reactions and EquationsDocument8 pagesChemical Reactions and EquationsDog100% (1)
- GCSE Exam QuestionsDocument155 pagesGCSE Exam Questionsdanielphilip68No ratings yet
- Physical and Chemical Change WorksheetDocument1 pagePhysical and Chemical Change Worksheetapi-350245383No ratings yet
- Grade 6 Chemistry Annual Term Revision WorksheetsDocument9 pagesGrade 6 Chemistry Annual Term Revision WorksheetsAbhayNo ratings yet
- (GR) COMPLETE - Multiple Choice Quiz Questions - Chemistry PDFDocument30 pages(GR) COMPLETE - Multiple Choice Quiz Questions - Chemistry PDFYasir AkhunNo ratings yet
- 4 - Unicellular Structural AdaptationsDocument12 pages4 - Unicellular Structural AdaptationsNayani Snigdha0% (1)
- Chemistry Final Exam For Grade 9Document5 pagesChemistry Final Exam For Grade 9debbie teferaNo ratings yet
- 5) Percent CompositionDocument11 pages5) Percent CompositionRamona Esteves100% (1)
- States of Matter: Paper 1: Practice TestDocument4 pagesStates of Matter: Paper 1: Practice TestSadaqat UllahNo ratings yet
- Quizzes 2Document3 pagesQuizzes 2api-254428474No ratings yet
- Practice Worksheet of Chemical BondingDocument2 pagesPractice Worksheet of Chemical Bondingch khakanNo ratings yet
- Elements, Compounds and MixturesDocument71 pagesElements, Compounds and MixturesAkshitSanghavi100% (1)
- Form 1 Chapter 3 - Three States of MatterDocument11 pagesForm 1 Chapter 3 - Three States of MatterMohd Safwan Mohd IsaNo ratings yet
- Physical and Chemical ChangeDocument10 pagesPhysical and Chemical ChangeAdrian CadizNo ratings yet
- Worksheet 1 - Chemical BondingDocument4 pagesWorksheet 1 - Chemical BondingFahd KhanNo ratings yet
- Long Quiz About Asexual and SexualDocument1 pageLong Quiz About Asexual and SexualLynne Tuiza EndonNo ratings yet
- Specialised CellsDocument34 pagesSpecialised CellsShaikBasheerAhmedNizami100% (1)
- Chapter Test On Sound WavesDocument1 pageChapter Test On Sound WavesryanmanubagNo ratings yet
- Separation Techniques Worksheet Ms Tay-1Document2 pagesSeparation Techniques Worksheet Ms Tay-1Anthony BensonNo ratings yet
- Pure Substances Vs MixturesDocument25 pagesPure Substances Vs Mixturesmisterbrowner100% (7)
- Name of Atom Common Ionic ChargeDocument2 pagesName of Atom Common Ionic ChargeMichael Rey MendozaNo ratings yet
- Separation TechniqueDocument8 pagesSeparation TechniqueachoeyzNo ratings yet
- Final Exam Gen. Chem1Document3 pagesFinal Exam Gen. Chem1Joemar GagnaoNo ratings yet
- Plant Reproduction QuizDocument3 pagesPlant Reproduction QuizLKM100% (1)
- THE PERIODIC TABLE - Multiple Choice Review QuestionsDocument5 pagesTHE PERIODIC TABLE - Multiple Choice Review Questionssaga_1150% (4)
- 1.2 Mole ConceptDocument66 pages1.2 Mole Conceptnurain syuhadaNo ratings yet
- Separation HW BOOK PDFDocument9 pagesSeparation HW BOOK PDFAngela KocevskaNo ratings yet
- Most Essential Learning CompetenciesDocument8 pagesMost Essential Learning CompetenciesKaterina TagleNo ratings yet
- Classification of Matter Worksheet #4: P A - C MDocument2 pagesClassification of Matter Worksheet #4: P A - C Mkaruro JorudenuNo ratings yet
- School Safety Assessment Tool For The Pilot Study On Face-To-Face of Learning ModalityDocument5 pagesSchool Safety Assessment Tool For The Pilot Study On Face-To-Face of Learning ModalityMon Agulto LomedaNo ratings yet
- Table 29. Number of Functional Computers in The School by Funding Source, Sy 2019-2020 (As of June 30, 2021)Document2 pagesTable 29. Number of Functional Computers in The School by Funding Source, Sy 2019-2020 (As of June 30, 2021)Mon Agulto LomedaNo ratings yet
- Project Proposal BONDPAPER 1Document1 pageProject Proposal BONDPAPER 1Mon Agulto LomedaNo ratings yet
- Project Proposal MURAL PAINTINGDocument2 pagesProject Proposal MURAL PAINTINGMon Agulto LomedaNo ratings yet
- Virgen Delas Flores High SchoolDocument2 pagesVirgen Delas Flores High SchoolMon Agulto LomedaNo ratings yet
- Virgen Delas Flores High SchoolDocument1 pageVirgen Delas Flores High SchoolMon Agulto LomedaNo ratings yet
- Schools Division of Bulacan: Virgen Dela Flores High SchoolDocument12 pagesSchools Division of Bulacan: Virgen Dela Flores High SchoolMon Agulto LomedaNo ratings yet
- Self Learning MaterialDocument39 pagesSelf Learning MaterialMon Agulto LomedaNo ratings yet
- Grade 8 Resectioning - AS OF SEPT. 12 PMDocument35 pagesGrade 8 Resectioning - AS OF SEPT. 12 PMMon Agulto LomedaNo ratings yet
- Grade 9 Resectioning List of Students Per Section UpdatedDocument13 pagesGrade 9 Resectioning List of Students Per Section UpdatedMon Agulto LomedaNo ratings yet
- Science - Grade 8 Supplementary Learning Resources Quarter IV: Roles of Organisms First Edition 2020Document23 pagesScience - Grade 8 Supplementary Learning Resources Quarter IV: Roles of Organisms First Edition 2020Mon Agulto LomedaNo ratings yet