Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
29 viewsMost Common: Transcribed By: Fatima Jen Pagsolingan
Most Common: Transcribed By: Fatima Jen Pagsolingan
Uploaded by
Kay Sabigan1) Radiation comes in two forms: ionizing radiation which can damage tissues by ionization, and non-ionizing radiation like radio waves which do not.
2) Common types of ionizing radiation are alpha particles, beta particles, gamma rays, x-rays, and neutrons. Alpha particles have a positive charge and short range, beta particles are electrons, gamma rays are electromagnetic waves, and neutrons have no charge.
3) Sources of radiation include nuclear reactors, medical equipment, naturally occurring elements like radon gas, and some building materials that contain radioactive elements in rocks and soil. Internal radiation exposure can occur from ingesting or inhaling radioactive substances.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Folio Radioactivity Ting 5Document16 pagesFolio Radioactivity Ting 5akunaruto92100% (3)
- Physical Chemistry PDFDocument390 pagesPhysical Chemistry PDFMbabazi RodneyNo ratings yet
- Radioactivity 2Document24 pagesRadioactivity 2John ProtoctisNo ratings yet
- Radiation Physics: PHY370 Physics Instrumentation and ApplicationDocument47 pagesRadiation Physics: PHY370 Physics Instrumentation and ApplicationaisyahNo ratings yet
- Radiation PollutionDocument15 pagesRadiation Pollutionkuya geekNo ratings yet
- Hiro 1Document6 pagesHiro 1Aron JaroNo ratings yet
- Radioactive RaysDocument11 pagesRadioactive RaysMita NurhayatiNo ratings yet
- Radiation Biolog1Document20 pagesRadiation Biolog1Degoma, Mary NoelynNo ratings yet
- Chem L1Document14 pagesChem L1ShannNo ratings yet
- 1-The Physics and Chemistry of Radiation AbsorptionDocument38 pages1-The Physics and Chemistry of Radiation AbsorptionliskatriNo ratings yet
- SCEICNEDocument6 pagesSCEICNESaren, Emil G.No ratings yet
- IONOZING RADIATIONDocument18 pagesIONOZING RADIATION230102912No ratings yet
- RPP Study MaterialDocument14 pagesRPP Study MaterialKartik SharmaNo ratings yet
- Nota Padat Fizik F5 RadioactivityDocument23 pagesNota Padat Fizik F5 Radioactivityslokkro97% (33)
- Radiation: Engr. Francia L. Abarientos Mechanical Engineering DepartmentDocument19 pagesRadiation: Engr. Francia L. Abarientos Mechanical Engineering DepartmentLucas Aldous MatthewNo ratings yet
- RadioactivityDocument7 pagesRadioactivitybrenda.mboghoNo ratings yet
- Doe Ionizing Radiation Dose Ranges Jan 2018Document8 pagesDoe Ionizing Radiation Dose Ranges Jan 2018Fabiane GarbimNo ratings yet
- Physics Edexcel Unit 3 - Radioactivity and AstronomyDocument44 pagesPhysics Edexcel Unit 3 - Radioactivity and Astronomyshivensolanki31No ratings yet
- 320-056 Ion FsDocument5 pages320-056 Ion FsViệt QuốcNo ratings yet
- RadiationDocument31 pagesRadiationFazly RahmanNo ratings yet
- Radioactivity: Submitted by T.Priya Dharshini CB19S076895 Iii Biotechnology - B Submitted To Dr.J.RevathyDocument7 pagesRadioactivity: Submitted by T.Priya Dharshini CB19S076895 Iii Biotechnology - B Submitted To Dr.J.RevathyPriya KarpagamNo ratings yet
- Lecture 5 - Radioactivity and Nuclear Chemistry - Updated by WWLDocument95 pagesLecture 5 - Radioactivity and Nuclear Chemistry - Updated by WWLchanchunho123456No ratings yet
- Mahmud RAHMAN 4.4.2.1 Radioactive Decay and Nuclear RadiationDocument18 pagesMahmud RAHMAN 4.4.2.1 Radioactive Decay and Nuclear RadiationMahmud RahmanNo ratings yet
- Radiation Physics IDocument73 pagesRadiation Physics IYustia FardaNo ratings yet
- RAD 104 Chapter - 09 Radiation Protection 2022 - 1Document41 pagesRAD 104 Chapter - 09 Radiation Protection 2022 - 1fadakm07No ratings yet
- RadiationDocument31 pagesRadiationS RahmnNo ratings yet
- Tracer TechniquesDocument61 pagesTracer Techniquesshruti shahNo ratings yet
- Radiopharmaceutics PDFDocument22 pagesRadiopharmaceutics PDFahmedesmail282005No ratings yet
- Science FolioDocument14 pagesScience FolioJunn KyleNo ratings yet
- Ionizing RadiationDocument18 pagesIonizing RadiationHyacinth Rae Ramirez Aranas - LipatNo ratings yet
- Types of Ionizing RadiationDocument6 pagesTypes of Ionizing RadiationApurva SrivastavaNo ratings yet
- Alpha Beta Gamma Particles: Electronic & Instrumentation EngineeringDocument9 pagesAlpha Beta Gamma Particles: Electronic & Instrumentation EngineeringShivam Kumar YadavNo ratings yet
- Radioactivity (Notes)Document23 pagesRadioactivity (Notes)Prakash JaisNo ratings yet
- Nuclear Physics CMDocument63 pagesNuclear Physics CMPeggyNo ratings yet
- RadioactivityDocument18 pagesRadioactivityAkaNayep ApNo ratings yet
- Radioacitvity Lesson 1Document25 pagesRadioacitvity Lesson 1tanishknandal2009No ratings yet
- TE - NORM Awarness - Eng.Document37 pagesTE - NORM Awarness - Eng.Tohamy MuhamadNo ratings yet
- Su5c14 by Adel KhamisDocument10 pagesSu5c14 by Adel KhamisAdel KhamisNo ratings yet
- Radbi Kelompok 2Document36 pagesRadbi Kelompok 2Try MutaqienNo ratings yet
- Radio Activity and ParticlesDocument34 pagesRadio Activity and ParticlesKiron SheiqNo ratings yet
- Nuclear PhysicsDocument55 pagesNuclear PhysicsMuhammad KhanNo ratings yet
- Center of Biomedical Engineering Medical Radiation Physics (Phys-2223)Document20 pagesCenter of Biomedical Engineering Medical Radiation Physics (Phys-2223)Sador YonasNo ratings yet
- Week 2 Lab - Alpha Beta Gamma LabDocument18 pagesWeek 2 Lab - Alpha Beta Gamma Labapi-747303766No ratings yet
- Training Manual 2 Radiation InjuriesDocument40 pagesTraining Manual 2 Radiation InjuriesAditi JainNo ratings yet
- 9.1radiographic Testing-Part1Document20 pages9.1radiographic Testing-Part1Mohanad AlmalahNo ratings yet
- Summary Notes - Topic 7 Radioactivity and Particles - Edexcel Physics IGCSEDocument5 pagesSummary Notes - Topic 7 Radioactivity and Particles - Edexcel Physics IGCSECollins JimNo ratings yet
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Handbook of Fluorescent Gems and Minerals - An Exposition and Catalog of the Fluorescent and Phosphorescent Gems and Minerals, Including the Use of Ultraviolet Light in the Earth SciencesFrom EverandHandbook of Fluorescent Gems and Minerals - An Exposition and Catalog of the Fluorescent and Phosphorescent Gems and Minerals, Including the Use of Ultraviolet Light in the Earth SciencesNo ratings yet
- Atoms in Motion: Understanding Nuclear Physics in Everyday LifeFrom EverandAtoms in Motion: Understanding Nuclear Physics in Everyday LifeNo ratings yet
- Proton Beam Radiotherapy: Physics and BiologyFrom EverandProton Beam Radiotherapy: Physics and BiologyKoji TsuboiNo ratings yet
- Electrodynamic Waves: Wireless Transmission Through Air, Metals, Water and the Human BodyFrom EverandElectrodynamic Waves: Wireless Transmission Through Air, Metals, Water and the Human BodyNo ratings yet
- The Fast Track to Understanding Ham Radio PropagationFrom EverandThe Fast Track to Understanding Ham Radio PropagationNo ratings yet
- Nature is a Powerhouse of Electricity! Physics Books for Kids | Children's Physics BooksFrom EverandNature is a Powerhouse of Electricity! Physics Books for Kids | Children's Physics BooksNo ratings yet
- SputteringDocument17 pagesSputteringTonmoy PaulNo ratings yet
- Fiitjee - Jee (Main) : Physics, Chemistry & MathematicsDocument14 pagesFiitjee - Jee (Main) : Physics, Chemistry & Mathematicsmanoj kumarNo ratings yet
- Arihant NEET Objective Physics Volume 2 by DC Pandey 2022 Edition (1) - Unlocked-Compressed-Part - 1Document200 pagesArihant NEET Objective Physics Volume 2 by DC Pandey 2022 Edition (1) - Unlocked-Compressed-Part - 1Mukul Kayal86% (14)
- Unit 4 - Electricity Quiz #10Document15 pagesUnit 4 - Electricity Quiz #10heyitsmemuahNo ratings yet
- Chemical BondingDocument8 pagesChemical BondingJoe AppiahNo ratings yet
- APES Units 1 & 2: Abiotic and Biotic Parts of EcosystemsDocument115 pagesAPES Units 1 & 2: Abiotic and Biotic Parts of EcosystemsJulisa HenryNo ratings yet
- PHYS 110 Test BankDocument27 pagesPHYS 110 Test BankSaudi ArabiaNo ratings yet
- General-Chemistry1 Quarter1 Week2Document24 pagesGeneral-Chemistry1 Quarter1 Week2Rose RepuestoNo ratings yet
- Atomic SturctureDocument9 pagesAtomic SturctureGaber HassanNo ratings yet
- Atom FactsDocument3 pagesAtom Factsaaditya panwarNo ratings yet
- Lecture Notes - Final PDFDocument40 pagesLecture Notes - Final PDFN KrishnaNo ratings yet
- Bishop Study Guide 7Document16 pagesBishop Study Guide 7Faisal Mohad Al SakhenNo ratings yet
- Electronic ConfigurationDocument19 pagesElectronic ConfigurationSheilavee Gatan TaguinodNo ratings yet
- Enrique Cavazos - Week1Grade7ScienceDocument25 pagesEnrique Cavazos - Week1Grade7ScienceEnrique CavazosNo ratings yet
- 2 Module Basic InstrumentationDocument33 pages2 Module Basic InstrumentationRyan LaRoqueNo ratings yet
- Atomic Structure History Dalton-BohrDocument28 pagesAtomic Structure History Dalton-BohrFabrielle RafaelNo ratings yet
- Atomic StructureDocument73 pagesAtomic StructureriomjNo ratings yet
- Electronic Partition Function Paradox: StricklerDocument3 pagesElectronic Partition Function Paradox: StricklerCarlos José Páez GonzálezNo ratings yet
- Nuclear Reactions: Rutherford's Alpha Scattering ExperimentDocument38 pagesNuclear Reactions: Rutherford's Alpha Scattering ExperimentSandesh R BhatNo ratings yet
- Periodic Table Periodic TrendDocument56 pagesPeriodic Table Periodic TrendJose AgresNo ratings yet
- Density of StatesDocument6 pagesDensity of States9810482818No ratings yet
- Lesson 3 THE PERIODIC TABLE AND ELECTRONIC STRUCTURE OF ATOMDocument13 pagesLesson 3 THE PERIODIC TABLE AND ELECTRONIC STRUCTURE OF ATOMscientistgenerosoNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument3 pagesProtons, Neutrons, and Electrons Practice WorksheetAriane Rosan Bocalan Ausmolo-DionisioNo ratings yet
- Geas 3Document5 pagesGeas 3Julio Gabriel AseronNo ratings yet
- Advanced Physical Chemistry AssignmentDocument2 pagesAdvanced Physical Chemistry AssignmentMosisa DugasaNo ratings yet
- Reviewer For 3rd QuarterDocument76 pagesReviewer For 3rd QuarterrhainbaguioroNo ratings yet
- DGFVSDVVZC Ries With AIATS For CBSE, NTSE & Olympiads 2024 - Class IXDocument22 pagesDGFVSDVVZC Ries With AIATS For CBSE, NTSE & Olympiads 2024 - Class IXAtul KunduNo ratings yet
- What Is Hydrogen BondingDocument6 pagesWhat Is Hydrogen BondingKate MagpayoNo ratings yet
- Class: M3 Subject: Chemistry Chapter 1: Basic Concepts of ChemistryDocument6 pagesClass: M3 Subject: Chemistry Chapter 1: Basic Concepts of Chemistrysamarth chawlaNo ratings yet
Most Common: Transcribed By: Fatima Jen Pagsolingan
Most Common: Transcribed By: Fatima Jen Pagsolingan
Uploaded by
Kay Sabigan0 ratings0% found this document useful (0 votes)
29 views4 pages1) Radiation comes in two forms: ionizing radiation which can damage tissues by ionization, and non-ionizing radiation like radio waves which do not.
2) Common types of ionizing radiation are alpha particles, beta particles, gamma rays, x-rays, and neutrons. Alpha particles have a positive charge and short range, beta particles are electrons, gamma rays are electromagnetic waves, and neutrons have no charge.
3) Sources of radiation include nuclear reactors, medical equipment, naturally occurring elements like radon gas, and some building materials that contain radioactive elements in rocks and soil. Internal radiation exposure can occur from ingesting or inhaling radioactive substances.
Original Description:
Radiation Bio notes
Original Title
Radiation Bio
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1) Radiation comes in two forms: ionizing radiation which can damage tissues by ionization, and non-ionizing radiation like radio waves which do not.
2) Common types of ionizing radiation are alpha particles, beta particles, gamma rays, x-rays, and neutrons. Alpha particles have a positive charge and short range, beta particles are electrons, gamma rays are electromagnetic waves, and neutrons have no charge.
3) Sources of radiation include nuclear reactors, medical equipment, naturally occurring elements like radon gas, and some building materials that contain radioactive elements in rocks and soil. Internal radiation exposure can occur from ingesting or inhaling radioactive substances.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
29 views4 pagesMost Common: Transcribed By: Fatima Jen Pagsolingan
Most Common: Transcribed By: Fatima Jen Pagsolingan
Uploaded by
Kay Sabigan1) Radiation comes in two forms: ionizing radiation which can damage tissues by ionization, and non-ionizing radiation like radio waves which do not.
2) Common types of ionizing radiation are alpha particles, beta particles, gamma rays, x-rays, and neutrons. Alpha particles have a positive charge and short range, beta particles are electrons, gamma rays are electromagnetic waves, and neutrons have no charge.
3) Sources of radiation include nuclear reactors, medical equipment, naturally occurring elements like radon gas, and some building materials that contain radioactive elements in rocks and soil. Internal radiation exposure can occur from ingesting or inhaling radioactive substances.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 4
University of the Philippines Manila o From x-ray machines
Biology 124 – Radiation Biology • *Most common
Prof. Marla Endriga
Radiation and Radioactivity Alpha (α) particles
Transcribed by: Fatima Jen Pagsolingan • Have positive electric charge
• Emitted from naturally-occurring elements such as
What is radiation? uranium and radium, as well as from some man-made
Solely produced by nuclear industry and nuclear radioactive elements
weapons • An alpha particle is made up of two protons and two
Any energy that is either transmitter or absorbed neutron bound together, therefore it is massive
Technically, energy in the form of particles or rays which An alpha particle is identical with the nucleus of a helium
travel through space atom
2 general types: non-ionizing and ionizing • Travel slowly, collide readily with matter and lose their
energy quickly
Non-ionizing Radiation o Linear Energy Transfer
• Exists in various forms o Radiation hits absorbing material. The energy
o Radio waves of radiation transfers to the absorbing material.
• Have little penetrating power and can be stopped by a
o Microwaves
sheet of paper or by the first layer of the skin and thus,
o Infrared rays
o Visible light present no external hazard
o Ultraviolet rays • If they are taken in the body, for example by breathing or
swallowing, alpha particles can affect the body’s cells
• Some are detected by our senses while other forms are
• Because they give up their energy over a relatively short
recognized through special instruments that convert his
type of radiation into signals that our senses can distance, alpha particles can inflict more biological
recognize damage inside the body than other kinds of radiation
• Polonium 210 – an alpha emitter
• Non-ionizing radiation wavelengths are longer than
o A Russian spy (Alexander Litvinenko) ate food
ionizing radiation wavelengths.
contaminated with Polonium 210. A few weeks
after ingestion, he died of radiation sickness.
o Its transport and access is highly regulated
across borders.
Beta (β) particles
• Negatively charged particles identical to electrons
• Much smaller, lighter and more penetrating than alpha
particles
o They can pass through 1 to 2 centimeters of
water and human flesh
o Beta particles are considered a slight external
hazard depending on their energy (low,
medium and high energy)
• Can be stopped by a sheet of aluminum a few millimeters
thick, by window glass, wood or a sheet of metal
Ionizing Radiation • Can also be hazardous if taken into the body
• Undetected by any of our physical senses o Hazard depends on dose and energy
• Can be detected with simple radiation detection • E.g. natural radioactivity from potassium-40 in soil,
instruments rocks and minerals; tritium, a hydrogen isotope
• Occurs in two forms (rays and particles) and at the high
frequency end of the electromagnetic spectrum Gamma (γ) rays
• Carries sufficient energy to knock electrons off other • Electromagnetic waves of very short wavelength
atoms – leaving them electrically charged or ionized traveling with the speed of light
• In living tissues, the ions caused by such radiation can o These are waves, alpha and beta are particlces.
affect normal biological processes o Speed of light – 3 x 108 m/s or 186,000 mi/s
• Because ions have an electrical charge, they are easy to • Have greater energy than medical x-rays
detect and makes it possible to measure the amount of • Emitted from the nuclei of some radioactive atoms (like
radiation present in extremely low levels uranium-238) when they decay
• Very penetrating and can pass right through the human
Common types of ionizing radiation body
• Emitted by the nuclei of radioactive atoms • Can be used in hospitals to diagnose and treat cancer or
• Alpha particles in industrial operations to detect defects in welds or
• Beta particles pipes
• Neutrons • Dense materials such as lead and concrete are excellent
• Gamma rays barriers against gamma rays
• X-radiation
o Not strictly nuclear in origin but is included X-rays
here as a form of ionizing radiation very similar • Electromagnetic waves with lesser energy and
to gamma radiation penetrating power than gamma rays
• Produced by x-ray machines when high-speed electrons o Building materials which use soil and rocks as
strike a metal target components also add measurable radiation
• Are used in many diagnostic procedures in medicine amounts
• Can penetrate the human body, but are absorbed by • Radon
denser tissues such as bone o This is a radioactive gas that naturally comes
o These show up as the white parts on an x-ray from the radioactive decay of radium
image o Can seep out from where they are produced
• X-rays that are not absorbed pass right through and into rocks or building materials of our homes,
cause the photographic film to darken when developed and can then be inhaled along with its decay
products
Neutrons o Radon and its decay products are the biggest
• Particles with no charge source of natural radiation in this world
• Can penetrate many materials very easily o It is significant enough that PNRI (Philippine
• Formed during the splitting (fission) of certain atoms Nuclear Research Institute) continuously
inside a nuclear reactor monitors radon levels throughout the country.
• Are not actually ionizing radiation, but if they hit other • Internal radiation (food and drink)
nuclei, they may cause emission of gamma rays or o Since radioactive materials occur everywhere
charged particles, indirectly giving rise to ionizing in nature, it is inevitable that they get into
radiation drinking water and food.
• More penetrating than gamma rays o Potassium-40 in particular is a major source of
• Can be stopped only by thick concrete, water or paraffin internal radiation.
barrier, or hydrogenous materials o The element potassium is essential to man,
plants and animals. Banana!
Sources of Ionizing Radiation o From plants, potassium finds its way through
• Natural food chains into man where it is built up in
o Background radiation body tissue, particularly muscle (C-14)
o Exist anywhere and everywhere, varying
intensity from place to place Artificial or Man – Made Sources of Ionizing Radiation
They do not increase unless there is a • Medical Applications
fallout. o The biggest (~90%) man-made contribution to
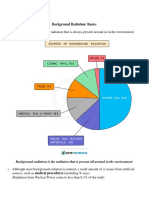
o Represents about 80% of all the radiation to radiation exposure of individual comes from
which we are exposed the medical and dental use of x-rays and from
• Man-made/Artificial radioactive materials used to diagnose or treat
o Doses received are much smaller than those diseases.
from natural radiation but they still vary o For some diseases, diagnostic information can
considerably be given by the gamma rays emitted by
o Only about 20% of man’s radiation exposure radioactive materials or those that are
comes from artificial or man-made radioactive surrounded into the patient by injection, or by
sources swallowing or by inhalation. (technique:
o Are in principle fully controllable, unlike Nuclear Medicine)
natural sources o Doses are carefully controlled.
o E.g. x-ray o Other medical applications include:
o Use of radioactive cobalt for the treatment of
Naturally-Occurring Sources of Radiation cancers
• Cosmic Radiation o Injection of radioactive iodine (Iodine 131)
o We are constantly bombarded by high sub- which concentrates in the thyroid for
atomic particles and gamma rays coming from treatment of goiters and thyroid disorders.
the sun and outer space • Radiation in Consumer Products
o As most of it is blocked by the earth’s o Minute radiation doses are received from
atmosphere, only a fraction of it reaches the artificial radioactivity in consumer products.
ground o Smoke detectors used radioactive Americium-
o The dose from cosmic radiation varies from 241 to detect smoke particles in the air.
one location to another depending on altitude, o In some luminous signs and gas mantles,
latitude and very occasionally on the solar cycle fluorescence and brightness are due to the
(sunspot) radioactive material.
• Earth’s Crust (Terrestrial Radiation) o The amount of radioactive material in these
o This radiation source comes from naturally- products are so small it does not have any effect
occurring radioactive elements that have on the consumer
existed since the earth was formed and are still • Fallout
around because they remain radioactive (they o Radioactive fallout from nuclear weapons tests
are still decaying) for many millions of years. carried out in the atmosphere is the most
o Main contributors: uranium-238, uranium- widespread environmental contaminant.
235, thorium-232, potassium-40 and their Doses to the public have declined
decay products which give off alpha and beta from relatively high values in the
particles (and sometimes gamma) early 1960s to very low levels in
continuously recent years.
o Where tests were carried out at ground level or The ion pair consists of a negatively-
even underground, localized contamination charged free electron and a
often remains near the weapons site. positively-charged atom
• Nuclear and Other Industries • Excitation
o Other artificial sources include small quantities o Occurs when an inner electron of an atom
of radioactive materials which can be released receives enough energy to allow it to move into
to the environment in the course of normal an excited stage at a higher energy level
operation of nuclear installations. o Transfer is only temporary
o Radioisotopes such as cobalt-60 are also used o The electron soon returns to its original level
in industry to sterilize products such as by re-emitting the energy gained as
cosmetics and medical supplies. electromagnetic radiation
o Other isotopes are used in nuclear gauges to
determine the thickness of materials such as Interaction of the Different Types of Ionizing Radiation with
paper products, plastic films and metal sheets. Matter
o Radiation is also utilized to measure liquid • Alpha particles
levels in large storage tanks and to determine • Beta Particles
the quality of welds in structures such as • Gamma & X-rays
bridges and buildings. o Photoelectric effect
o Compton effect
Global Radiation Dose o Pair production
Exposure of people from natural sources is about 80%
and about 20% from artificial sources (Data from the US) Interaction of Alpha Particles with Matter
Unit: Sievert (Sv) • Massive
o Each particle consists of two protons and two
neutrons and travel relatively slowly through
matter
• Have high chance of interaction with atoms along its path
and will give up some of its energy during each
interaction
o Lose energy very rapidly and only travel very
short distances in dense materials
• Mainly lose their energy in matter through the process of
ionization and excitation
How does radiation interact with matter?
• All ionizing radiation emit energy either in the form of
rays (x-rays, gamma rays) or particles (alpha, beta,
neutrons)
• It transfers energy to the electrons of the absorbing
material
o The energy transfer may occur by several
mechanisms Interaction of Beta Particles with Matter
o The two most commonly encountered are: • Identical to the orbital electrons of atoms in the
ionization and excitation absorbing material
• Much smaller compared to alpha particles and travel
General Mechanisms of Absorption/Transfer of Energy in much faster
Matter • Undergo fewer interactions per unit length of track and
• Ionization give up energy more slowly than alpha particles
o The energy transferred to the electrons causes • Travel farther than alpha particles in dense materials
them to be pulled away or removed from an • Also lose energy by excitation and ionization
atom entirely. • Some beta particles (particularly those with higher
May natatanggal na electron sa energy) that travel close to the nucleus of an atom
absorbing material and you may ions experience an attractive force causing them to deflect
produced. and lose energy with the production of x-rays
o The result is the formation of the so-called ion o Bremsstrahlung – braking radiation;
pair. secondary radiation
• Electron with positive charge: positron (as seen in beta o The angle at which a photon is scattered
plus decay) depends on its original energy and the energy
transferred to the electron
Two Types of Beta Decay: o Predominates in materials with higher atomic
number
• Pair production
o When a photon with energy of at least 1.02 MeV
passes near the nucleus, the energetic photon
may be converted into a positron and an
electron
o The positron and electron then lose kinetic
energy through interaction with other atoms in
the material
A positron cannot exist without
kinetic energy so when it has lost all
its energy, it will combine with an
electron of the absorbing material in
a process called annihilation
o Annihilation – two particles destroy each
other and are converted into two annihilation
photons, each of 0.51 MeV
These photons are emitted in
opposite directions from each other
o The most likely interaction for high energy
photons in materials with high atomic number
What units are used to measure radiation?
• Becquerel (Bq)
o In measuring radioactivity, Bq is used to
describe the number of nuclei which decay or
disintegrate in a second
o One Becquerel = 1 disintegration per second
The former unit used for this quantity
is the curie (Ci)
1 Ci = 37 billion Bq
Interaction of X-Rays and Gamma Rays with Matter
• Travel long distances between interactions
• Their energy cannot be completely absorbed, only
reduced in intensity
Three principal ways in which x-rays and gamma rays interact
with matter (depending on energy of radiation)
• Photoelectric effect
o A photon (gamma ray or x-ray) of relatively low
energy (< 1 MeV) transfers all its energy to a
tightly bound electron in an inner electron shell
or orbit
o Electron is then ejected from the atom with
considerable velocity – produces light
o Most likely to occur in materials with a high
atomic number, so a material such as lead (Z-
82) makes a useful shielding material for low
energy photons
• Compton Scattering
o Occurs when a medium energy photon (0.2 to 5
MeV) transfers part of its energy to a free or
loosely bound electron in the outermost shell of
an atom
o The electron from one of the outer shells is
released from the atom and the photon is
scattered with reduced energy
You might also like
- Folio Radioactivity Ting 5Document16 pagesFolio Radioactivity Ting 5akunaruto92100% (3)
- Physical Chemistry PDFDocument390 pagesPhysical Chemistry PDFMbabazi RodneyNo ratings yet
- Radioactivity 2Document24 pagesRadioactivity 2John ProtoctisNo ratings yet
- Radiation Physics: PHY370 Physics Instrumentation and ApplicationDocument47 pagesRadiation Physics: PHY370 Physics Instrumentation and ApplicationaisyahNo ratings yet
- Radiation PollutionDocument15 pagesRadiation Pollutionkuya geekNo ratings yet
- Hiro 1Document6 pagesHiro 1Aron JaroNo ratings yet
- Radioactive RaysDocument11 pagesRadioactive RaysMita NurhayatiNo ratings yet
- Radiation Biolog1Document20 pagesRadiation Biolog1Degoma, Mary NoelynNo ratings yet
- Chem L1Document14 pagesChem L1ShannNo ratings yet
- 1-The Physics and Chemistry of Radiation AbsorptionDocument38 pages1-The Physics and Chemistry of Radiation AbsorptionliskatriNo ratings yet
- SCEICNEDocument6 pagesSCEICNESaren, Emil G.No ratings yet
- IONOZING RADIATIONDocument18 pagesIONOZING RADIATION230102912No ratings yet
- RPP Study MaterialDocument14 pagesRPP Study MaterialKartik SharmaNo ratings yet
- Nota Padat Fizik F5 RadioactivityDocument23 pagesNota Padat Fizik F5 Radioactivityslokkro97% (33)
- Radiation: Engr. Francia L. Abarientos Mechanical Engineering DepartmentDocument19 pagesRadiation: Engr. Francia L. Abarientos Mechanical Engineering DepartmentLucas Aldous MatthewNo ratings yet
- RadioactivityDocument7 pagesRadioactivitybrenda.mboghoNo ratings yet
- Doe Ionizing Radiation Dose Ranges Jan 2018Document8 pagesDoe Ionizing Radiation Dose Ranges Jan 2018Fabiane GarbimNo ratings yet
- Physics Edexcel Unit 3 - Radioactivity and AstronomyDocument44 pagesPhysics Edexcel Unit 3 - Radioactivity and Astronomyshivensolanki31No ratings yet
- 320-056 Ion FsDocument5 pages320-056 Ion FsViệt QuốcNo ratings yet
- RadiationDocument31 pagesRadiationFazly RahmanNo ratings yet
- Radioactivity: Submitted by T.Priya Dharshini CB19S076895 Iii Biotechnology - B Submitted To Dr.J.RevathyDocument7 pagesRadioactivity: Submitted by T.Priya Dharshini CB19S076895 Iii Biotechnology - B Submitted To Dr.J.RevathyPriya KarpagamNo ratings yet
- Lecture 5 - Radioactivity and Nuclear Chemistry - Updated by WWLDocument95 pagesLecture 5 - Radioactivity and Nuclear Chemistry - Updated by WWLchanchunho123456No ratings yet
- Mahmud RAHMAN 4.4.2.1 Radioactive Decay and Nuclear RadiationDocument18 pagesMahmud RAHMAN 4.4.2.1 Radioactive Decay and Nuclear RadiationMahmud RahmanNo ratings yet
- Radiation Physics IDocument73 pagesRadiation Physics IYustia FardaNo ratings yet
- RAD 104 Chapter - 09 Radiation Protection 2022 - 1Document41 pagesRAD 104 Chapter - 09 Radiation Protection 2022 - 1fadakm07No ratings yet
- RadiationDocument31 pagesRadiationS RahmnNo ratings yet
- Tracer TechniquesDocument61 pagesTracer Techniquesshruti shahNo ratings yet
- Radiopharmaceutics PDFDocument22 pagesRadiopharmaceutics PDFahmedesmail282005No ratings yet
- Science FolioDocument14 pagesScience FolioJunn KyleNo ratings yet
- Ionizing RadiationDocument18 pagesIonizing RadiationHyacinth Rae Ramirez Aranas - LipatNo ratings yet
- Types of Ionizing RadiationDocument6 pagesTypes of Ionizing RadiationApurva SrivastavaNo ratings yet
- Alpha Beta Gamma Particles: Electronic & Instrumentation EngineeringDocument9 pagesAlpha Beta Gamma Particles: Electronic & Instrumentation EngineeringShivam Kumar YadavNo ratings yet
- Radioactivity (Notes)Document23 pagesRadioactivity (Notes)Prakash JaisNo ratings yet
- Nuclear Physics CMDocument63 pagesNuclear Physics CMPeggyNo ratings yet
- RadioactivityDocument18 pagesRadioactivityAkaNayep ApNo ratings yet
- Radioacitvity Lesson 1Document25 pagesRadioacitvity Lesson 1tanishknandal2009No ratings yet
- TE - NORM Awarness - Eng.Document37 pagesTE - NORM Awarness - Eng.Tohamy MuhamadNo ratings yet
- Su5c14 by Adel KhamisDocument10 pagesSu5c14 by Adel KhamisAdel KhamisNo ratings yet
- Radbi Kelompok 2Document36 pagesRadbi Kelompok 2Try MutaqienNo ratings yet
- Radio Activity and ParticlesDocument34 pagesRadio Activity and ParticlesKiron SheiqNo ratings yet
- Nuclear PhysicsDocument55 pagesNuclear PhysicsMuhammad KhanNo ratings yet
- Center of Biomedical Engineering Medical Radiation Physics (Phys-2223)Document20 pagesCenter of Biomedical Engineering Medical Radiation Physics (Phys-2223)Sador YonasNo ratings yet
- Week 2 Lab - Alpha Beta Gamma LabDocument18 pagesWeek 2 Lab - Alpha Beta Gamma Labapi-747303766No ratings yet
- Training Manual 2 Radiation InjuriesDocument40 pagesTraining Manual 2 Radiation InjuriesAditi JainNo ratings yet
- 9.1radiographic Testing-Part1Document20 pages9.1radiographic Testing-Part1Mohanad AlmalahNo ratings yet
- Summary Notes - Topic 7 Radioactivity and Particles - Edexcel Physics IGCSEDocument5 pagesSummary Notes - Topic 7 Radioactivity and Particles - Edexcel Physics IGCSECollins JimNo ratings yet
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- Handbook of Fluorescent Gems and Minerals - An Exposition and Catalog of the Fluorescent and Phosphorescent Gems and Minerals, Including the Use of Ultraviolet Light in the Earth SciencesFrom EverandHandbook of Fluorescent Gems and Minerals - An Exposition and Catalog of the Fluorescent and Phosphorescent Gems and Minerals, Including the Use of Ultraviolet Light in the Earth SciencesNo ratings yet
- Atoms in Motion: Understanding Nuclear Physics in Everyday LifeFrom EverandAtoms in Motion: Understanding Nuclear Physics in Everyday LifeNo ratings yet
- Proton Beam Radiotherapy: Physics and BiologyFrom EverandProton Beam Radiotherapy: Physics and BiologyKoji TsuboiNo ratings yet
- Electrodynamic Waves: Wireless Transmission Through Air, Metals, Water and the Human BodyFrom EverandElectrodynamic Waves: Wireless Transmission Through Air, Metals, Water and the Human BodyNo ratings yet
- The Fast Track to Understanding Ham Radio PropagationFrom EverandThe Fast Track to Understanding Ham Radio PropagationNo ratings yet
- Nature is a Powerhouse of Electricity! Physics Books for Kids | Children's Physics BooksFrom EverandNature is a Powerhouse of Electricity! Physics Books for Kids | Children's Physics BooksNo ratings yet
- SputteringDocument17 pagesSputteringTonmoy PaulNo ratings yet
- Fiitjee - Jee (Main) : Physics, Chemistry & MathematicsDocument14 pagesFiitjee - Jee (Main) : Physics, Chemistry & Mathematicsmanoj kumarNo ratings yet
- Arihant NEET Objective Physics Volume 2 by DC Pandey 2022 Edition (1) - Unlocked-Compressed-Part - 1Document200 pagesArihant NEET Objective Physics Volume 2 by DC Pandey 2022 Edition (1) - Unlocked-Compressed-Part - 1Mukul Kayal86% (14)
- Unit 4 - Electricity Quiz #10Document15 pagesUnit 4 - Electricity Quiz #10heyitsmemuahNo ratings yet
- Chemical BondingDocument8 pagesChemical BondingJoe AppiahNo ratings yet
- APES Units 1 & 2: Abiotic and Biotic Parts of EcosystemsDocument115 pagesAPES Units 1 & 2: Abiotic and Biotic Parts of EcosystemsJulisa HenryNo ratings yet
- PHYS 110 Test BankDocument27 pagesPHYS 110 Test BankSaudi ArabiaNo ratings yet
- General-Chemistry1 Quarter1 Week2Document24 pagesGeneral-Chemistry1 Quarter1 Week2Rose RepuestoNo ratings yet
- Atomic SturctureDocument9 pagesAtomic SturctureGaber HassanNo ratings yet
- Atom FactsDocument3 pagesAtom Factsaaditya panwarNo ratings yet
- Lecture Notes - Final PDFDocument40 pagesLecture Notes - Final PDFN KrishnaNo ratings yet
- Bishop Study Guide 7Document16 pagesBishop Study Guide 7Faisal Mohad Al SakhenNo ratings yet
- Electronic ConfigurationDocument19 pagesElectronic ConfigurationSheilavee Gatan TaguinodNo ratings yet
- Enrique Cavazos - Week1Grade7ScienceDocument25 pagesEnrique Cavazos - Week1Grade7ScienceEnrique CavazosNo ratings yet
- 2 Module Basic InstrumentationDocument33 pages2 Module Basic InstrumentationRyan LaRoqueNo ratings yet
- Atomic Structure History Dalton-BohrDocument28 pagesAtomic Structure History Dalton-BohrFabrielle RafaelNo ratings yet
- Atomic StructureDocument73 pagesAtomic StructureriomjNo ratings yet
- Electronic Partition Function Paradox: StricklerDocument3 pagesElectronic Partition Function Paradox: StricklerCarlos José Páez GonzálezNo ratings yet
- Nuclear Reactions: Rutherford's Alpha Scattering ExperimentDocument38 pagesNuclear Reactions: Rutherford's Alpha Scattering ExperimentSandesh R BhatNo ratings yet
- Periodic Table Periodic TrendDocument56 pagesPeriodic Table Periodic TrendJose AgresNo ratings yet
- Density of StatesDocument6 pagesDensity of States9810482818No ratings yet
- Lesson 3 THE PERIODIC TABLE AND ELECTRONIC STRUCTURE OF ATOMDocument13 pagesLesson 3 THE PERIODIC TABLE AND ELECTRONIC STRUCTURE OF ATOMscientistgenerosoNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument3 pagesProtons, Neutrons, and Electrons Practice WorksheetAriane Rosan Bocalan Ausmolo-DionisioNo ratings yet
- Geas 3Document5 pagesGeas 3Julio Gabriel AseronNo ratings yet
- Advanced Physical Chemistry AssignmentDocument2 pagesAdvanced Physical Chemistry AssignmentMosisa DugasaNo ratings yet
- Reviewer For 3rd QuarterDocument76 pagesReviewer For 3rd QuarterrhainbaguioroNo ratings yet
- DGFVSDVVZC Ries With AIATS For CBSE, NTSE & Olympiads 2024 - Class IXDocument22 pagesDGFVSDVVZC Ries With AIATS For CBSE, NTSE & Olympiads 2024 - Class IXAtul KunduNo ratings yet
- What Is Hydrogen BondingDocument6 pagesWhat Is Hydrogen BondingKate MagpayoNo ratings yet
- Class: M3 Subject: Chemistry Chapter 1: Basic Concepts of ChemistryDocument6 pagesClass: M3 Subject: Chemistry Chapter 1: Basic Concepts of Chemistrysamarth chawlaNo ratings yet