Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
23 viewsApoptosis and Caspases
Apoptosis and Caspases
Uploaded by
Kunhutty Vasudevan AnubhamaCaspases are cysteine proteases that cleave proteins at aspartate residues, triggering apoptosis. They are synthesized as inactive pro-caspases and activated through proteolytic cleavage, often in cascades where one caspase activates another. The extrinsic pathway is triggered by death ligands binding to cell surface receptors, recruiting an adaptor protein complex containing pro-caspase-8 which activates it. Caspase-8 then activates downstream executioner caspases. The intrinsic pathway involves mitochondrial outer membrane permeabilization releasing proteins like cytochrome c and caspase-9, which activate the apoptosome and caspase cascade. Both pathways activate executioner caspases like caspase-3 leading to apoptosis.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Cell InjuryDocument118 pagesCell InjuryShanzayNo ratings yet
- The Nervous SystemDocument71 pagesThe Nervous SystemDennis Limosnero MayorNo ratings yet
- Review Mechanisms of Caspase Activation and Inhibition During ApoptosisDocument12 pagesReview Mechanisms of Caspase Activation and Inhibition During ApoptosisFery WirawanNo ratings yet
- The Biology of Apoptosis: Fouad Boulos, MD August 2010Document4 pagesThe Biology of Apoptosis: Fouad Boulos, MD August 2010ans11No ratings yet
- Principles of Diuretic Therapy: Dr. Rania Magadmi, MBBS, PHDDocument24 pagesPrinciples of Diuretic Therapy: Dr. Rania Magadmi, MBBS, PHDمشاعرمبعثرةNo ratings yet
- Review Article Immunotherapy in The Treatment of Metastatic Melanoma: Current Knowledge and Future DirectionsDocument16 pagesReview Article Immunotherapy in The Treatment of Metastatic Melanoma: Current Knowledge and Future DirectionsSeprianto RuslanNo ratings yet
- Drugs in LactationDocument36 pagesDrugs in Lactationcorzpun168678100% (1)
- Diuretic DrugsDocument2 pagesDiuretic DrugsEngku ElisaNo ratings yet
- Bcl-2 Family ProteinsDocument25 pagesBcl-2 Family Proteinsማላያላም ማላያላም100% (1)
- Drug Toxicity in Pregnancy and LactationDocument3 pagesDrug Toxicity in Pregnancy and LactationPardeep SonyNo ratings yet
- ABT-199, A Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing PlateletsDocument9 pagesABT-199, A Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing PlateletsSanziana CristeaNo ratings yet
- Drug Use During Pregnancy and LactationDocument45 pagesDrug Use During Pregnancy and LactationSayli GoreNo ratings yet
- Biomarkers in MelanomaDocument6 pagesBiomarkers in MelanomaMatthew NgNo ratings yet
- Cell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartDocument20 pagesCell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartHumbertoNo ratings yet
- Signal Transduction & G Protein-Coupled Receptors: TopicsDocument33 pagesSignal Transduction & G Protein-Coupled Receptors: TopicsEria MarinaNo ratings yet
- 15 Diuretic AgentsDocument50 pages15 Diuretic AgentsAngelyn Si100% (1)
- Ligand and Cell Signaling PathwaysDocument117 pagesLigand and Cell Signaling PathwaysShoaib MomenNo ratings yet
- Metabolic Biochemistry, Volume - T. P. MommsenDocument511 pagesMetabolic Biochemistry, Volume - T. P. MommsenJesus Daniel Morales MarquezNo ratings yet
- Drug Transport Across Cell Membrane: Dr. Salman H. RizviDocument67 pagesDrug Transport Across Cell Membrane: Dr. Salman H. RizviIrum RafeeqNo ratings yet
- 9 Anti PlateletDocument15 pages9 Anti PlateletHely PatelNo ratings yet
- Cell SignallingDocument72 pagesCell SignallingSheerin SulthanaNo ratings yet
- ErgotDocument40 pagesErgotAna Luca100% (1)
- Fac Thrombolysis 2007Document36 pagesFac Thrombolysis 2007Tri Harjono0% (1)
- Pancreas 2021Document32 pagesPancreas 2021Dr. AliNo ratings yet
- Cell SignalingDocument53 pagesCell SignalingKaushiki KalravNo ratings yet
- Protein Folding - IDocument3 pagesProtein Folding - IJENIFER PEARLIN0% (1)
- G-protein-Coupled ReceptorsDocument24 pagesG-protein-Coupled ReceptorsNaimi Amalia hatimahNo ratings yet
- CH 11 PPT Cell CommunicationDocument46 pagesCH 11 PPT Cell CommunicationpalakNo ratings yet
- 6-8 Thrombolitics Antiplatelet AnticoagulantDocument36 pages6-8 Thrombolitics Antiplatelet AnticoagulantFatima ZahraNo ratings yet
- Cell-Mediated Immune Responses8Document26 pagesCell-Mediated Immune Responses8Trifu AndreiNo ratings yet
- Prostate TumorsDocument17 pagesProstate TumorsElian Jesús JimenezNo ratings yet
- The Biological Effect of NanoparticlesDocument16 pagesThe Biological Effect of NanoparticlesmihaelaputinaNo ratings yet
- Pharmaco KineticsDocument16 pagesPharmaco KineticsanaeshklNo ratings yet
- DNA TopoisomerasesDocument61 pagesDNA TopoisomerasesBabak NamiNo ratings yet
- Cell Mediated Immune ResponsesDocument21 pagesCell Mediated Immune ResponsesStefan AlexandruNo ratings yet
- MHC I Proteins, Which Present Antigens To Cytotoxic T Cells, (2) MHC II Proteins, Which Present Antigens T Helper CellsDocument25 pagesMHC I Proteins, Which Present Antigens To Cytotoxic T Cells, (2) MHC II Proteins, Which Present Antigens T Helper CellsMudassar Roomi100% (1)
- Functional Human Physiology: For The Exercise and Sport Sciences The Cardiovascular System: Cardiac FunctionDocument186 pagesFunctional Human Physiology: For The Exercise and Sport Sciences The Cardiovascular System: Cardiac FunctionBery Agana F. PurbaNo ratings yet
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- RIBOZYMESDocument17 pagesRIBOZYMESSharan GayathrinathanNo ratings yet
- Big Question: What Happens When Immune System Overreacts?Document42 pagesBig Question: What Happens When Immune System Overreacts?Louloun MoussignacNo ratings yet
- Drug Use in Pregnancy and LactationDocument16 pagesDrug Use in Pregnancy and LactationkarotisNo ratings yet
- Neurogenic and Myogenic HeartsDocument14 pagesNeurogenic and Myogenic HeartsSantanu100% (1)
- Serotonin (5-HT)Document35 pagesSerotonin (5-HT)adeesasaadNo ratings yet
- Drug Use During Pregnancy and LactationDocument50 pagesDrug Use During Pregnancy and LactationJuveria Fatima75% (4)
- The Complement SystemDocument24 pagesThe Complement Systemhkatniwala100% (1)
- GluconeogenesisDocument11 pagesGluconeogenesisMithilesh RautNo ratings yet
- Cytochrome P450 ChartDocument2 pagesCytochrome P450 ChartCristinaNo ratings yet
- Vo2 Max Lab Report DoneDocument6 pagesVo2 Max Lab Report Doneapi-547193352No ratings yet
- Pharmacology PDFDocument109 pagesPharmacology PDFRanjit TharuNo ratings yet
- Body System ChecklistDocument6 pagesBody System Checklistapi-422967453No ratings yet
- Chromatin RemodellingDocument234 pagesChromatin Remodellingplastioid4079No ratings yet
- Hematopoiesis DiagramDocument1 pageHematopoiesis DiagramApril Rose Airoso - AramburoNo ratings yet
- Advanced Biochemistry SeriesDocument373 pagesAdvanced Biochemistry SeriesProf Rakesh SharmaNo ratings yet
- Chapter 21: Introduction To Pharmacology of CNS DrugsDocument19 pagesChapter 21: Introduction To Pharmacology of CNS DrugsJoslin Roz GalileaNo ratings yet
- ProteinDocument13 pagesProteinapi-272775120No ratings yet
- Mechanisms of Hormone Action NotesDocument3 pagesMechanisms of Hormone Action Notesapi-390361165No ratings yet
- Principles of Flow CytometryDocument6 pagesPrinciples of Flow CytometryMohammad Abul FarahNo ratings yet
- Introduction To Endocrinology For Clinical StudentsDocument28 pagesIntroduction To Endocrinology For Clinical StudentsOhwovoriole ToketemuNo ratings yet
- Cell SignallingDocument37 pagesCell SignallingSreekarWunnava100% (1)
- Membranes for Life SciencesFrom EverandMembranes for Life SciencesKlaus-Viktor PeinemannNo ratings yet
- Mitochondria and ChloroplastDocument71 pagesMitochondria and ChloroplastRifai Alfarabi100% (1)
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- Apoptosis Lecture 2023 2024Document115 pagesApoptosis Lecture 2023 2024Thái AnNo ratings yet
- Apoptotic and Non-Apoptotic Cell DeathDocument186 pagesApoptotic and Non-Apoptotic Cell DeathKoaLa AyuNdhaNo ratings yet
- Coprinus Comatus (Higher Basidiomycetes) ExtractDocument10 pagesCoprinus Comatus (Higher Basidiomycetes) ExtractDodo BabyNo ratings yet
- 1 s2.0 S0045206818311969 MainDocument20 pages1 s2.0 S0045206818311969 MainLuiz Guilherme FiorinNo ratings yet
- 1 s2.0 S2468054023000239 MainDocument9 pages1 s2.0 S2468054023000239 MainSyarifa Syafira Al BahasyimNo ratings yet
- Apoptosis Cytotoxicity and Cell ProliferationDocument186 pagesApoptosis Cytotoxicity and Cell ProliferationsauravsarkarNo ratings yet
- Programmed Cell Death in AgingDocument25 pagesProgrammed Cell Death in AgingGustavo Sá MottaNo ratings yet
- Intreeso To ApoptossisDocument9 pagesIntreeso To ApoptossisToh Qin KaneNo ratings yet
- Promega Timing Apoptosis Assay PublicationDocument4 pagesPromega Timing Apoptosis Assay Publicationαγαπημένη του ΧριστούNo ratings yet
- Cell Death in Biology and Diseases: Series EditorsDocument411 pagesCell Death in Biology and Diseases: Series Editorsmaria100% (1)
- Manuscript FiksDocument7 pagesManuscript Fiksninik_suprayitnoNo ratings yet
- Hypersensitive Response in PlantsDocument18 pagesHypersensitive Response in PlantsMehak MattooNo ratings yet
- Kuk M.Sc. Zoology 1st Sem Notes PDFDocument327 pagesKuk M.Sc. Zoology 1st Sem Notes PDFyuikoNo ratings yet
- Apoptosis: Protocols & Applications Guide Rev. 1/06Document23 pagesApoptosis: Protocols & Applications Guide Rev. 1/06Fidiya Septi Kusma WardaniNo ratings yet
- The Apoptosis in The CancerDocument260 pagesThe Apoptosis in The Cancerqasim_saeed_1100% (1)
- Apoptosis Poster March 20106547Document1 pageApoptosis Poster March 20106547Achmad JunaidiNo ratings yet
- Understanding Apoptosis and Apoptotic Pathways Targeted CancerDocument14 pagesUnderstanding Apoptosis and Apoptotic Pathways Targeted Canceranupama_rani_2No ratings yet
- The Reactome BookDocument3,252 pagesThe Reactome BookA RoyNo ratings yet
- Mitochondrial Membrane PermeabilizationDocument66 pagesMitochondrial Membrane PermeabilizationGus Tavo BrugesNo ratings yet
Apoptosis and Caspases
Apoptosis and Caspases
Uploaded by
Kunhutty Vasudevan Anubhama0 ratings0% found this document useful (0 votes)
23 views19 pagesCaspases are cysteine proteases that cleave proteins at aspartate residues, triggering apoptosis. They are synthesized as inactive pro-caspases and activated through proteolytic cleavage, often in cascades where one caspase activates another. The extrinsic pathway is triggered by death ligands binding to cell surface receptors, recruiting an adaptor protein complex containing pro-caspase-8 which activates it. Caspase-8 then activates downstream executioner caspases. The intrinsic pathway involves mitochondrial outer membrane permeabilization releasing proteins like cytochrome c and caspase-9, which activate the apoptosome and caspase cascade. Both pathways activate executioner caspases like caspase-3 leading to apoptosis.
Original Description:
Role of Apoptosis and Caspases in Cancer
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCaspases are cysteine proteases that cleave proteins at aspartate residues, triggering apoptosis. They are synthesized as inactive pro-caspases and activated through proteolytic cleavage, often in cascades where one caspase activates another. The extrinsic pathway is triggered by death ligands binding to cell surface receptors, recruiting an adaptor protein complex containing pro-caspase-8 which activates it. Caspase-8 then activates downstream executioner caspases. The intrinsic pathway involves mitochondrial outer membrane permeabilization releasing proteins like cytochrome c and caspase-9, which activate the apoptosome and caspase cascade. Both pathways activate executioner caspases like caspase-3 leading to apoptosis.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
23 views19 pagesApoptosis and Caspases
Apoptosis and Caspases
Uploaded by
Kunhutty Vasudevan AnubhamaCaspases are cysteine proteases that cleave proteins at aspartate residues, triggering apoptosis. They are synthesized as inactive pro-caspases and activated through proteolytic cleavage, often in cascades where one caspase activates another. The extrinsic pathway is triggered by death ligands binding to cell surface receptors, recruiting an adaptor protein complex containing pro-caspase-8 which activates it. Caspase-8 then activates downstream executioner caspases. The intrinsic pathway involves mitochondrial outer membrane permeabilization releasing proteins like cytochrome c and caspase-9, which activate the apoptosome and caspase cascade. Both pathways activate executioner caspases like caspase-3 leading to apoptosis.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 19
Apoptosis and the Caspases
• Caspases are specific proteases that act like molecular scissors to
cleave intracellular proteins at aspartate residues.
• The term “caspases” derives from three of the characteristics of the
enzymes: they are cysteine-rich aspartate proteases.
• They are synthesized as inactive enzymes called pro-caspases that
need to be cleaved at aspartate residues in order to be activated.
• Although for the most part pro-caspases are considered inactive, pro-
caspases possess some activity (about 2% of the proteolytic activity of
fully activated caspases).
• This may seem insignificant at the moment but, it is an important
feature for some pathways of caspase activation.
• Moreover, as caspases cleave at aspartate residues and pro-caspases
are themselves activated by cleavage at aspartate residues, caspases
participate in a cascade of activation whereby one caspase can activate
another caspase in a chain reaction.
• This mechanism, whereby caspases activate procaspases, leads to
amplification of an apoptotic signal
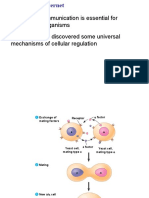
Simple Caspase activation pathway
Role in extrinsic pathway
The extrinsic pathway of apoptosis
• Death signals, TNF and FAS, activate their death receptors TNF receptor
and FAS receptor respectively.
• Binding causes a change in shape and oligomerization of the receptors.
• Adaptor proteins recognize the activated receptors and lead to the
aggregation of procaspase 8.
• The function of adaptor proteins is to transduce the death signal from the
receptor to caspases.
• The adaptors recruit several molecules of procaspase-8 via death effector
domains (DED).
• Procaspase aggregation leads to caspase 8 activation.
• Caspase-8 is known as an initiator caspase as it is the first link between
the receptor and the apoptotic proteases and it is key to the extrinsic
pathway.
• This initiator caspase initiates a caspase cascade, followed by proteolysis,
and apoptosis.
• Molecules of procaspase-8, now in close proximity to each other,
become activated by self-cleavage as procaspases have low
enzymatic activity.
• Together the death ligands, receptors, adaptors, and initiator
caspase are called the death inducing signaling complex (DISC).
• Caspase-8 initiates a cascade of caspase activation: one activated
caspase cleaves and activates other caspases, called executioner
caspases (caspase-3, -6, and -7).
• The cascade ultimately causes the cleavage of specific protein
targets and results in apoptosis.
• This process can be inhibited by c-Flip, an inhibitor of apoptosis. It
can inhibit the interactions of procaspase 8 adaptors. It binds to
adaptor FADD via a DED and inhibit caspase-8 recruitment and
activation.
• The breakdown of the cell results from the proteolysis of the target
proteins.
• Target proteins include nuclear lamins allowing for nuclear
shrinkage, cytoskeletal proteins such as actin and intermediate
filaments for rearranging cell structure, specific kinases for cell
signaling, and other enzymes such as caspase-activated DNase for
the cleavage of chromatin.
• The caspase-activated DNase cuts DNA between nucleosomes and
generates a DNA ladder (corresponding to multiples of 180 bp—the
distance between nucleosomes), that can be detected
experimentally and is used by scientists as a molecular marker of
apoptosis (TUNEL technique).
• Caspases also cleave the tumor suppressor protein, RB, and this
cleavage results in the degradation of RB protein.
• This event is required for apoptosis induced by TNF and points to a
role for RB in the inhibition of apoptosis.
Role in intrinsic pathway
The intrinsic pathway of Apoptosis
• Cell stress triggers the BH3 only protein, Bid, to transiently bind to
and activate Bax.
• Bax undergoes a conformational change, inserts into the outer
mitochondrial membrane and oligomerizes (6 – 8 molecules).
• Important regulators are released from the intermembrane space.
• Cytochrome c and pro-caspase 9 join Apaf-1 to form the
Apoptosome.
• Caspase aggregation leads to the activation of procaspase 9 and
finally a caspase cascade.
• Second mitochondria derived activator (Smac/DIABLO), also
released from the mitochondria, inhibits Inhibitors of Apoptosis
proteins (IAP) that normally act to block caspases.
• The Bcl-2 family of proteins is thought to act either by forming
channels to promote apoptosis or blocking the BH3 domains of pro-
apoptotic proteins to inhibit apoptosis.
• It is the balance of these two activities that regulates the release of
important molecular apoptotic mediators from the mitochondria.
• The members of this family can associate by protein–protein
interactions and it appears that it is the ratio of activity that
determines function and hence the outcome.
• For example, if the activity of the pro-apoptotic factors is high
owing to low inhibition from anti-apoptotic factors, apoptosis is
triggered.
• The activity of the proteins of the Bcl-2 family can also be regulated
by phosphorylation.
• The intermembrane space between the two mitochondrial
membranes acts as a supply cabinet for apoptotic mediators.
• The pro-apoptotic Bcl-2 members regulate the release of the apoptotic
mediators from this mitochondrial compartment in a process
sometimes referred to as mitochondrial outer membrane
permeabilization (MOMP).
• Upon activation by an apoptotic signal, BH3 only proteins, Bid and Bim,
bind to and activate Bax.
• This interaction is transient and induces a conformational change in
Bax as it translocates from the cytoplasm to the mitochondria, and
inserts into the outer mitochondrial membrane and oligomerizes.
• This increases the permeability of the outer mitochondrial membrane
by forming membrane channels and allows the release of apoptotic
mediators.
• The BH3 domain of Bax is required for its killing activity and
interactions with anti-apoptotic proteins.
• Cytochrome c, which also functions in the electron transport chain of
aerobic respiration, and procaspase-9 are released into the cytoplasm
and assemble into a complex called an apoptosome, along with dATP
bound to Apaf-1.
• The binding of cytochrome c to cytosolic Apaf-1 triggers the formation
of a wheel-like heptameric complex that facilitates the recruitment of
procaspase-9 via protein domains, called CARD domains, present on
both Apaf-1 and procaspase-9.
• Recent structural studies suggest that the CARD domains, and
therefore procaspase-9 molecules, reside in a central ring.
• Further, cytochrome c binds to Apaf-1 within clefts formed by a pair of
β propellers at the end of the spoke-like helical domain of Apaf-1 with
a 1:1 stoichiometry.
• Apaf-1 is a protein co-factor that is required for activation of
procaspase-9.
• Caspase-9 is an initiator caspase activated by pro-caspase aggregation
that begins another caspase cascade activating downstream caspases-
3, -6, and -7. Thus, caspase-9 is key to the intrinsic pathway.
• Other factors, such as inhibitors of apoptosis proteins (IAPs; eight
mammalian IAPs have been identified) and Smac (second mitochondria
derived activator)/DIABLO play a role in modulating the process.
• The X-chromosome linked member, XIAP, is one member of the IAP family
that directly binds to and inhibits the activity of caspase-3 and caspase-7,
after they have been processed, by binding to their active site.
• XIAP also inhibits caspase-9, but does so by binding to monomeric
caspase-9 and locking the active site in an aberrant conformation.
• A transcription factor called NFκB, a major player in inflammation, is a
potent inhibitor of apoptosis. It induces the transcription of IAPs.
• Smac/DIABLO, another regulator released from the mitochondria,
eliminates inhibition by IAPs. Smac/DIABLO competes with activated
caspase-9 for binding to XIAP.
• Both caspase-9 and Smac contain a similar tetrapeptide domain that binds
to XIAP.
• Thus, opposing effects on caspase activity are regulated by conserved IAP-
binding motifs in caspase-9 and Smac.
Cross-talk between extrinsic and
intrinsic pathways
• Bid, a pro-apoptotic Bcl-2 family member
links the intrinsic and extrinsic pathways
of apoptosis.
• Bid can stimulate the intrinsic pathway of
apoptosis by
– directly activating Bax and Bak,
– facilitating the release of
cytochrome c from the
mitochondria, and
– inducing the subsequent activation
of downstream caspases.
• In addition, Bid links the intrinsic pathway
with the regulation of cell cycle
progression in response to DNA damage.
• Phosphorylation of Bid by ATM kinase is
required for cell cycle arrest in response
to DNA damage
MITOCHONDRIAL DEATH
SIGNALING PATHWAYS
The extrinsic pathway: mediated by
membrane death receptors
• A death factor such as Fas ligand or tumor necrosis factor (TNF) is received
by a transmembrane death receptor such as Fas receptor or TNF receptor,
respectively.
• TNF is a soluble factor while Fas ligand is bound to the plasma membrane
of neighboring cells.
• When ligands bind to the death receptors, the receptors undergo a
conformational change and oligomerize in order to transduce the signal
into the cell.
• The conformational change exposes so-called death domains that are
located on the receptors’ cytoplasmic tail and enable intracellular adaptor
proteins such as FADD (Fas-associated death domain protein) and TRADD
(TNF receptor-associated death domain protein) to bind via their death
domains.
• A death domain is part of a protein, approximately 70 amino acids, that
allows for specific protein–protein interactions to occur and is analogous
to the SH2 domain characteristic of growth factor signal transduction
pathways.
TRAIL receptors
• A subfamily of TNF receptors, called TRAIL receptors (TRAILR1 and
TRAILR2; also know as death receptor 4 and 5—DR4, DR5), has been found
to elicit a differential sensitivity to apoptosis between normal cells and
cancer cells.
• Its ligand, TRAIL (TNF-related apoptosis-inducing ligand), induces
apoptosis in many cancer cells (regardless of the p53 gene profile) but not
in most normal cells.
• This subfamily signals apoptosis in a similar manner to TNF receptors
recruiting adaptors (e.g. FADD) and an initiator caspase, caspase-8, to the
membrane.
• As TRAIL and its receptors are expressed in most organs, it is hypothesized
that the addition or loss of regulatory molecules determines whether
apoptosis will be induced in particular cell types.
• The differences identified between normal and tumor cells create an
opportunity for designing drugs that target the apoptotic pathways and
suggest that attempts to restore apoptotic activity will not affect normal
cells.
Differences in caspase activation and response to
TRAIL in normal versus cancer cells
Applications
• Mutations in death receptor genes, such as those encoding
the Fas receptor and TRAIL receptor, occur in some cancers.
• Fas ligand and receptor are induced by UV light.
• Signaling through Fas receptors induces the development
of sunburn in response to UV and is an important defense
against skin cancers (Guzman et al., 2003).
• Somatic mutations in Fas receptors have been reported in
melanomas and squamous cell carcinomas.
• Some types of chemotherapy induce particular cells of the
immune system to produce TNF, thus triggering the
extrinsic pathway.
You might also like
- Cell InjuryDocument118 pagesCell InjuryShanzayNo ratings yet
- The Nervous SystemDocument71 pagesThe Nervous SystemDennis Limosnero MayorNo ratings yet
- Review Mechanisms of Caspase Activation and Inhibition During ApoptosisDocument12 pagesReview Mechanisms of Caspase Activation and Inhibition During ApoptosisFery WirawanNo ratings yet
- The Biology of Apoptosis: Fouad Boulos, MD August 2010Document4 pagesThe Biology of Apoptosis: Fouad Boulos, MD August 2010ans11No ratings yet
- Principles of Diuretic Therapy: Dr. Rania Magadmi, MBBS, PHDDocument24 pagesPrinciples of Diuretic Therapy: Dr. Rania Magadmi, MBBS, PHDمشاعرمبعثرةNo ratings yet
- Review Article Immunotherapy in The Treatment of Metastatic Melanoma: Current Knowledge and Future DirectionsDocument16 pagesReview Article Immunotherapy in The Treatment of Metastatic Melanoma: Current Knowledge and Future DirectionsSeprianto RuslanNo ratings yet
- Drugs in LactationDocument36 pagesDrugs in Lactationcorzpun168678100% (1)
- Diuretic DrugsDocument2 pagesDiuretic DrugsEngku ElisaNo ratings yet
- Bcl-2 Family ProteinsDocument25 pagesBcl-2 Family Proteinsማላያላም ማላያላም100% (1)
- Drug Toxicity in Pregnancy and LactationDocument3 pagesDrug Toxicity in Pregnancy and LactationPardeep SonyNo ratings yet
- ABT-199, A Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing PlateletsDocument9 pagesABT-199, A Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing PlateletsSanziana CristeaNo ratings yet
- Drug Use During Pregnancy and LactationDocument45 pagesDrug Use During Pregnancy and LactationSayli GoreNo ratings yet
- Biomarkers in MelanomaDocument6 pagesBiomarkers in MelanomaMatthew NgNo ratings yet
- Cell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartDocument20 pagesCell Signaling in Space and Time:: Where Proteins Come Together and When They're ApartHumbertoNo ratings yet
- Signal Transduction & G Protein-Coupled Receptors: TopicsDocument33 pagesSignal Transduction & G Protein-Coupled Receptors: TopicsEria MarinaNo ratings yet
- 15 Diuretic AgentsDocument50 pages15 Diuretic AgentsAngelyn Si100% (1)
- Ligand and Cell Signaling PathwaysDocument117 pagesLigand and Cell Signaling PathwaysShoaib MomenNo ratings yet
- Metabolic Biochemistry, Volume - T. P. MommsenDocument511 pagesMetabolic Biochemistry, Volume - T. P. MommsenJesus Daniel Morales MarquezNo ratings yet
- Drug Transport Across Cell Membrane: Dr. Salman H. RizviDocument67 pagesDrug Transport Across Cell Membrane: Dr. Salman H. RizviIrum RafeeqNo ratings yet
- 9 Anti PlateletDocument15 pages9 Anti PlateletHely PatelNo ratings yet
- Cell SignallingDocument72 pagesCell SignallingSheerin SulthanaNo ratings yet
- ErgotDocument40 pagesErgotAna Luca100% (1)
- Fac Thrombolysis 2007Document36 pagesFac Thrombolysis 2007Tri Harjono0% (1)
- Pancreas 2021Document32 pagesPancreas 2021Dr. AliNo ratings yet
- Cell SignalingDocument53 pagesCell SignalingKaushiki KalravNo ratings yet
- Protein Folding - IDocument3 pagesProtein Folding - IJENIFER PEARLIN0% (1)
- G-protein-Coupled ReceptorsDocument24 pagesG-protein-Coupled ReceptorsNaimi Amalia hatimahNo ratings yet
- CH 11 PPT Cell CommunicationDocument46 pagesCH 11 PPT Cell CommunicationpalakNo ratings yet
- 6-8 Thrombolitics Antiplatelet AnticoagulantDocument36 pages6-8 Thrombolitics Antiplatelet AnticoagulantFatima ZahraNo ratings yet
- Cell-Mediated Immune Responses8Document26 pagesCell-Mediated Immune Responses8Trifu AndreiNo ratings yet
- Prostate TumorsDocument17 pagesProstate TumorsElian Jesús JimenezNo ratings yet
- The Biological Effect of NanoparticlesDocument16 pagesThe Biological Effect of NanoparticlesmihaelaputinaNo ratings yet
- Pharmaco KineticsDocument16 pagesPharmaco KineticsanaeshklNo ratings yet
- DNA TopoisomerasesDocument61 pagesDNA TopoisomerasesBabak NamiNo ratings yet
- Cell Mediated Immune ResponsesDocument21 pagesCell Mediated Immune ResponsesStefan AlexandruNo ratings yet
- MHC I Proteins, Which Present Antigens To Cytotoxic T Cells, (2) MHC II Proteins, Which Present Antigens T Helper CellsDocument25 pagesMHC I Proteins, Which Present Antigens To Cytotoxic T Cells, (2) MHC II Proteins, Which Present Antigens T Helper CellsMudassar Roomi100% (1)
- Functional Human Physiology: For The Exercise and Sport Sciences The Cardiovascular System: Cardiac FunctionDocument186 pagesFunctional Human Physiology: For The Exercise and Sport Sciences The Cardiovascular System: Cardiac FunctionBery Agana F. PurbaNo ratings yet
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- RIBOZYMESDocument17 pagesRIBOZYMESSharan GayathrinathanNo ratings yet
- Big Question: What Happens When Immune System Overreacts?Document42 pagesBig Question: What Happens When Immune System Overreacts?Louloun MoussignacNo ratings yet
- Drug Use in Pregnancy and LactationDocument16 pagesDrug Use in Pregnancy and LactationkarotisNo ratings yet
- Neurogenic and Myogenic HeartsDocument14 pagesNeurogenic and Myogenic HeartsSantanu100% (1)
- Serotonin (5-HT)Document35 pagesSerotonin (5-HT)adeesasaadNo ratings yet
- Drug Use During Pregnancy and LactationDocument50 pagesDrug Use During Pregnancy and LactationJuveria Fatima75% (4)
- The Complement SystemDocument24 pagesThe Complement Systemhkatniwala100% (1)
- GluconeogenesisDocument11 pagesGluconeogenesisMithilesh RautNo ratings yet
- Cytochrome P450 ChartDocument2 pagesCytochrome P450 ChartCristinaNo ratings yet
- Vo2 Max Lab Report DoneDocument6 pagesVo2 Max Lab Report Doneapi-547193352No ratings yet
- Pharmacology PDFDocument109 pagesPharmacology PDFRanjit TharuNo ratings yet
- Body System ChecklistDocument6 pagesBody System Checklistapi-422967453No ratings yet
- Chromatin RemodellingDocument234 pagesChromatin Remodellingplastioid4079No ratings yet
- Hematopoiesis DiagramDocument1 pageHematopoiesis DiagramApril Rose Airoso - AramburoNo ratings yet
- Advanced Biochemistry SeriesDocument373 pagesAdvanced Biochemistry SeriesProf Rakesh SharmaNo ratings yet
- Chapter 21: Introduction To Pharmacology of CNS DrugsDocument19 pagesChapter 21: Introduction To Pharmacology of CNS DrugsJoslin Roz GalileaNo ratings yet
- ProteinDocument13 pagesProteinapi-272775120No ratings yet
- Mechanisms of Hormone Action NotesDocument3 pagesMechanisms of Hormone Action Notesapi-390361165No ratings yet
- Principles of Flow CytometryDocument6 pagesPrinciples of Flow CytometryMohammad Abul FarahNo ratings yet
- Introduction To Endocrinology For Clinical StudentsDocument28 pagesIntroduction To Endocrinology For Clinical StudentsOhwovoriole ToketemuNo ratings yet
- Cell SignallingDocument37 pagesCell SignallingSreekarWunnava100% (1)
- Membranes for Life SciencesFrom EverandMembranes for Life SciencesKlaus-Viktor PeinemannNo ratings yet
- Mitochondria and ChloroplastDocument71 pagesMitochondria and ChloroplastRifai Alfarabi100% (1)
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- Apoptosis Lecture 2023 2024Document115 pagesApoptosis Lecture 2023 2024Thái AnNo ratings yet
- Apoptotic and Non-Apoptotic Cell DeathDocument186 pagesApoptotic and Non-Apoptotic Cell DeathKoaLa AyuNdhaNo ratings yet
- Coprinus Comatus (Higher Basidiomycetes) ExtractDocument10 pagesCoprinus Comatus (Higher Basidiomycetes) ExtractDodo BabyNo ratings yet
- 1 s2.0 S0045206818311969 MainDocument20 pages1 s2.0 S0045206818311969 MainLuiz Guilherme FiorinNo ratings yet
- 1 s2.0 S2468054023000239 MainDocument9 pages1 s2.0 S2468054023000239 MainSyarifa Syafira Al BahasyimNo ratings yet
- Apoptosis Cytotoxicity and Cell ProliferationDocument186 pagesApoptosis Cytotoxicity and Cell ProliferationsauravsarkarNo ratings yet
- Programmed Cell Death in AgingDocument25 pagesProgrammed Cell Death in AgingGustavo Sá MottaNo ratings yet
- Intreeso To ApoptossisDocument9 pagesIntreeso To ApoptossisToh Qin KaneNo ratings yet
- Promega Timing Apoptosis Assay PublicationDocument4 pagesPromega Timing Apoptosis Assay Publicationαγαπημένη του ΧριστούNo ratings yet
- Cell Death in Biology and Diseases: Series EditorsDocument411 pagesCell Death in Biology and Diseases: Series Editorsmaria100% (1)
- Manuscript FiksDocument7 pagesManuscript Fiksninik_suprayitnoNo ratings yet
- Hypersensitive Response in PlantsDocument18 pagesHypersensitive Response in PlantsMehak MattooNo ratings yet
- Kuk M.Sc. Zoology 1st Sem Notes PDFDocument327 pagesKuk M.Sc. Zoology 1st Sem Notes PDFyuikoNo ratings yet
- Apoptosis: Protocols & Applications Guide Rev. 1/06Document23 pagesApoptosis: Protocols & Applications Guide Rev. 1/06Fidiya Septi Kusma WardaniNo ratings yet
- The Apoptosis in The CancerDocument260 pagesThe Apoptosis in The Cancerqasim_saeed_1100% (1)
- Apoptosis Poster March 20106547Document1 pageApoptosis Poster March 20106547Achmad JunaidiNo ratings yet
- Understanding Apoptosis and Apoptotic Pathways Targeted CancerDocument14 pagesUnderstanding Apoptosis and Apoptotic Pathways Targeted Canceranupama_rani_2No ratings yet
- The Reactome BookDocument3,252 pagesThe Reactome BookA RoyNo ratings yet
- Mitochondrial Membrane PermeabilizationDocument66 pagesMitochondrial Membrane PermeabilizationGus Tavo BrugesNo ratings yet