Professional Documents
Culture Documents
B49CE - Tutorial Topic 3 Questions v3
B49CE - Tutorial Topic 3 Questions v3
Uploaded by
BuyuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
B49CE - Tutorial Topic 3 Questions v3
B49CE - Tutorial Topic 3 Questions v3
Uploaded by
BuyuCopyright:
Available Formats
Tutorial Topic 3 Questions 1
3A.7 Tutorial 3
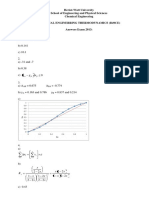
1. Use Raoult’s law to evaluate vapour-liquid equilibrium data for the
water-phenol system at 1 bar. Compare your calculated data with the
x y diagram provided (below) and comment on the assumptions
implied in the method of calculation.
B
log 10 ( Psat ) A P sat (mmHg) T (C)
T C
Component A B C
Water 8.10765 1750.226 235.000
Phenol 7.13301 1516.790 174.954
(Answer: see summary answers)
2. Benzene and toluene form an ideal liquid solution. Calculate the
compositions of the vapour and liquid in equilibrium at 760 mmHg total
pressure and at temperatures of 80oC, 92oC, 100oC and 110.4oC.
Vapour Pressure Data (mmHg)
Temperature (oC) Benzene Toluene
80.0 760 300
92.0 1078 432
100.0 1344 559
110.4 1748 760
(Answer: see summary answers)
©HERIOT-W ATT UNIVERSITY B49CE December 2017 v3
Tutorial Topic 3 Questions 2
3. Using the Van Laar equations, calculate the x y equilibrium
compositions at atmospheric pressure for a mixture of chloroform and
acetone. Chloroform and acetone form an azeotrope containing 0.666
mole fraction of chloroform at 64.5oC.
Vapour Pressure Data (mmHg)
Temperature (oC) Acetone Chloroform
45.0 510.5 439.0
50.0 612.6 526.0
55.0 --- 625.2
56.3 760.0 ---
60.0 860.6 739.2
60.9 --- 760.0
70.0 1190.0 1019.0
80.0 1611.0 1403.0
(Answer: see summary answers)
4. Experimental investigation shows that at 100.7oC and 760 mmHg, a
liquid of mole fractions 0.568 n-heptane and 0.432 toluene exists in
equilibrium with a vapour of mole fractions 0.637 n-heptane and 0.363
toluene.
Use Margule’s model to evaluate the composition of vapour in
equilibrium with a liquid containing mole fractions of 0.185 n-heptane
and 0.815 toluene.
Vapour pressure data at 100.7oC:
n-heptane 780 mmHg
toluene 432 mmHg
(Answer: y1 = 0.4347 and y2 = 0.5653)
5. Di-chloroethane and methanol form an azeotrope at 760 mmHg and
41.9oC containing a di-chloroethane mole fraction of 0.769. A
laboratory experiment carried out at 760 mmHg gave the following
equilibrium point:
x DCE 0.294 and y DCE 0.653
(Cont…..)
©HERIOT-W ATT UNIVERSITY B49CE December 2017 v3
Tutorial Topic 3 Questions 3
Use the azeotrope data and the Van Laar equations to predict the
composition of the vapour in equilibrium with the liquid above (0.294
mole fraction). Compare the predicted and experimental data and
comment on their accuracy.
B
log 10 ( Psat ) A
T C
In which P sat (mmHg) and T (C)
Component A B C
Di-chloroethane 6.96513 1141.984 231.93
Methanol 7.87863 1473.110 230.00
(Answer: y1 = 0.711, Comment….)
6. Using a laboratory still operating at 760 mmHg, experimental vapour-
liquid equilibrium data for the propanol-water system has been found
for two different liquid compositions as shown below:
x (Propanol) y (Propanol) Temperature (oC)
0.36 0.42 87.7
0.52 0.44 87.9
Using the Van Laar equations evaluate the activity coefficients of both
components and hence the vapour composition in equilibrium with a
liquid with a propanol mole fraction of 0.60 at 760 mmHg.
B
log 10 ( Psat ) A
T C
In which P sat (mmHg) and T (C).
Component A B C
Water (1) 7.96681 1668.21 228.0
Propanol (2) 7.99733 1569.70 209.5
(Answer: 1 2.001, 2 1.148 , y1 = 0.518 and y2 = 0.482)
©HERIOT-W ATT UNIVERSITY B49CE December 2017 v3
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5820)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- B49CE - Tutorial Topic 1 Questions v3Document2 pagesB49CE - Tutorial Topic 1 Questions v3BuyuNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Tutorial Answers - Separation B Tutorial - Combined Tutorial SolutionsDocument38 pagesTutorial Answers - Separation B Tutorial - Combined Tutorial SolutionsBuyuNo ratings yet
- Tutorial Answers - Separation B Tutorial - Combined Tutorial Solutions PDFDocument38 pagesTutorial Answers - Separation B Tutorial - Combined Tutorial Solutions PDFBuyuNo ratings yet
- Kato30t nk250 PDFDocument4 pagesKato30t nk250 PDFhakimi83100% (1)
- B49CE - Tutorial Topic 2 Questions v3Document6 pagesB49CE - Tutorial Topic 2 Questions v3BuyuNo ratings yet
- F17XB2 2014-15 Class Test Mathematics For Engineers and Scientists 2 Attempt All Questions (B)Document2 pagesF17XB2 2014-15 Class Test Mathematics For Engineers and Scientists 2 Attempt All Questions (B)BuyuNo ratings yet
- 1. A) Ln (F/P) = - 1/Rt ∫Αdp B) 0.141 C) 10.1 2. A) -31 And -7 B) 0.38 C) 3. A) A =-0.675 A = -0.774 B) Y = 0.163 And 0.786 Y = 0.837 And 0.214 C)Document1 page1. A) Ln (F/P) = - 1/Rt ∫Αdp B) 0.141 C) 10.1 2. A) -31 And -7 B) 0.38 C) 3. A) A =-0.675 A = -0.774 B) Y = 0.163 And 0.786 Y = 0.837 And 0.214 C)BuyuNo ratings yet
- Naming Organic Compounds B17CADocument2 pagesNaming Organic Compounds B17CABuyuNo ratings yet
- 2012 Dec SolutionsDocument8 pages2012 Dec SolutionsBuyu100% (1)
- 2014 DecDocument5 pages2014 DecBuyuNo ratings yet
- Torque Using in Installation For HSBDocument1 pageTorque Using in Installation For HSBKeith CuberoNo ratings yet
- Light - RefractionDocument18 pagesLight - RefractionSURESHNo ratings yet
- Unit-4 Inverse ResponseDocument3 pagesUnit-4 Inverse ResponseHilary TendaiNo ratings yet
- 16 Atomic Structure SolutionDocument34 pages16 Atomic Structure SolutionVINOD JINo ratings yet
- Evaluation of Non-Linear Normal ModesDocument9 pagesEvaluation of Non-Linear Normal ModesEmerson Borges SantanaNo ratings yet
- Chapter 1 - Introduction To Machinery PrinciplesDocument51 pagesChapter 1 - Introduction To Machinery PrinciplesGopinath SubramaniNo ratings yet
- NDT Newco Catalogo VARIOSDocument297 pagesNDT Newco Catalogo VARIOSDylanNo ratings yet
- Maintenance and Operation of Air Compressor PlantsDocument118 pagesMaintenance and Operation of Air Compressor Plantstenderncare100% (2)
- Manuel D'instructions Manuale Di Istruzioni Instruction ManualDocument72 pagesManuel D'instructions Manuale Di Istruzioni Instruction Manualanilr008No ratings yet
- G CO A 5 Symmetry PDFDocument3 pagesG CO A 5 Symmetry PDFAdel OmarNo ratings yet
- Determination of Plasticity Characteristics of Soils (Obe)Document15 pagesDetermination of Plasticity Characteristics of Soils (Obe)Suranga GayanNo ratings yet
- 2a KeyDocument16 pages2a KeyHimanshu RanjanNo ratings yet
- PIPE by Capote - TermsDocument109 pagesPIPE by Capote - TermsRochelle May CatbaganNo ratings yet
- Design and Analysis of U-Shaped Ribbon Blender With Screw ConveyorDocument9 pagesDesign and Analysis of U-Shaped Ribbon Blender With Screw ConveyorAasawari MahagaonkarNo ratings yet
- Thermowell and Immersion Probe Installation OverviewDocument7 pagesThermowell and Immersion Probe Installation OverviewBEN ADEGBULUNo ratings yet
- Exam 2021Document2 pagesExam 2021Tannaz HadizadeNo ratings yet
- Research ProposalDocument6 pagesResearch ProposalLoukit KhemkaNo ratings yet
- Miller Indices ResourceDocument34 pagesMiller Indices ResourceRamb23No ratings yet
- Dynamic Design Analysis Method (DDAM) With Abaqus 2005Document1 pageDynamic Design Analysis Method (DDAM) With Abaqus 2005SIMULIACorpNo ratings yet
- United States Patent 19 11 Patent Number: 5,697,468: Russell, Jr. Et Al. (45) Date of Patent: Dec. 16, 1997Document8 pagesUnited States Patent 19 11 Patent Number: 5,697,468: Russell, Jr. Et Al. (45) Date of Patent: Dec. 16, 1997155No ratings yet
- Application of Chitosan Shells Meti Batissa ViolacDocument12 pagesApplication of Chitosan Shells Meti Batissa Violacnia herianiNo ratings yet
- Polymer Brushes Via Surface-Initiated Controlled Radical PolymerizationDocument91 pagesPolymer Brushes Via Surface-Initiated Controlled Radical PolymerizationP'Parot Pornlapas100% (1)
- ResistorsDocument9 pagesResistorsanon_26317821No ratings yet
- SS316 Thermal ExpansionDocument4 pagesSS316 Thermal ExpansionCheng Khie ChiehNo ratings yet
- Data Visualization Tableau Dashboard - Empowering Business For Effective InsightsDocument8 pagesData Visualization Tableau Dashboard - Empowering Business For Effective InsightsGladin Koickaleth VargheseNo ratings yet
- Gas Turbine Generator: Chapter-7Document6 pagesGas Turbine Generator: Chapter-7GAGANNo ratings yet
- Tempmatic Climate ControlDocument144 pagesTempmatic Climate ControlGreg HannaNo ratings yet
- 43.6 Electric Circuits CIE IGCSE Physics Practical QPDocument11 pages43.6 Electric Circuits CIE IGCSE Physics Practical QPThe lonely ninjaNo ratings yet
- LET Reviewer General Education Part 3Document10 pagesLET Reviewer General Education Part 3Richelle WskiNo ratings yet