Professional Documents
Culture Documents
Department of Education Region VIII Schools Division of Tacloban City District Learning Center IV Leyte National High School Tacloban City
Department of Education Region VIII Schools Division of Tacloban City District Learning Center IV Leyte National High School Tacloban City
Uploaded by
Annabelle Cebrero Paulo AlbaoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Department of Education Region VIII Schools Division of Tacloban City District Learning Center IV Leyte National High School Tacloban City
Department of Education Region VIII Schools Division of Tacloban City District Learning Center IV Leyte National High School Tacloban City
Uploaded by
Annabelle Cebrero Paulo AlbaoCopyright:
Available Formats
Department of Education
Region VIII
Schools Division of Tacloban City
District Learning Center IV
LEYTE NATIONAL HIGH SCHOOL
Tacloban City
GENERAL CHEMISTRY II
QUIZ 4.1 Answer the following problem/s to the best of your ability:
1. (25 points) Provided the reaction A + 2B + C 2D + E, and the set of data that follows:
Experiment no. A B C rate
1 1.4 1.4 1.00 R1 = 1
2 0.7 1.4 1.00 1
R2 = 2
3 0.7 0.7 1.00 2
R3 = 4
4 1.4 1.4 0.50 R4 = 16R3
wherein the rate law is identified as rate = k [A]x [B]y [C]z, 1.1 what are the reaction orders with respect to A, B,
and C?; 1.2 what is the value of R5 in terms of the variable R1?
2. (5 points) what are the other terms for a transition state? Give the two other terms.
3. Identify whether which of the factors affecting rates of reaction does each of the following cases pertain to.
Write C concentration of substrate, T for temperature, P for physical states of the substrate, and Ct for addition
of catalyst.
3.1 (2 pts) pulverizing the solid solute before addition of the liquid solvent
3.2 (2 pts) adding salt to preserve meat
3.3 (2 pts) use of liquid nitrogen for “fried ice cream”
3.4 (2 pts) applying thick bleach to remove bathroom wall stains
3.5 (2 pts) use of confectioner’s sugar instead of table sugar for baking purposes
4. (5 pts) The rate of consumption of iodide, I-(aq), in the reaction:
S2O82- (aq) + 3I- (aq) I3- (aq) + 2SO42- (aq) is 5.40 (mol I-) L-1s-1. What is the rate of formation
of SO42-(aq)?
5. For items 5.1 to 5.2, write Aldim if the statement is correct and Roda if not.
5.1 (2 pts) The rate constant, k, depends on the order of reaction and the temperature.
5.2 (2 pts) The rate constant, k, depends on the nature of reactants and the temperature.
5.3 (3 pts) Ammonia, NH3, reacts with O2 to form NO and H2O as follows:
4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g)
At the instant when NH3 is reacting at a rate of 0.80 M/min, what is the rate at which O 2 is
disappearing?
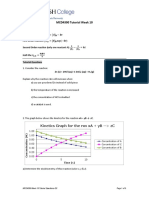
5.4 (3 pts) For the following reaction profile, indicate (a) the positions of reactants and products, (b) the
activation energy, (c) ΔE for the reaction.
Compiled by: ldddj.rch/2018
Compiled by: ldddj.rch/2018
You might also like
- Memorial City Mall MapDocument1 pageMemorial City Mall Maptruechris89No ratings yet
- Chem162 FractCryst Report Gradescope 021919 PCDocument18 pagesChem162 FractCryst Report Gradescope 021919 PCAmalawa Aiwekhoe0% (5)
- Chemistry 31 - Quantitative Analysis Exam #2, April 20, 2011Document5 pagesChemistry 31 - Quantitative Analysis Exam #2, April 20, 2011Agatha BermudezNo ratings yet
- Ventilator Waveform AnalysisDocument76 pagesVentilator Waveform Analysischtbht100% (1)
- SAI VRAT STORY KathaDocument2 pagesSAI VRAT STORY KathaRohit100% (2)
- CHE555 Assignment 2Document2 pagesCHE555 Assignment 2Eiman UzmiNo ratings yet
- NW NSC GR 11 Maths Lit P1 Eng Memo Nov 2019Document7 pagesNW NSC GR 11 Maths Lit P1 Eng Memo Nov 2019Kamo RagolaneNo ratings yet
- Kutlwanong 1st Full p2 2021 PDFDocument31 pagesKutlwanong 1st Full p2 2021 PDFhlayisofilesNo ratings yet
- 09 (2) PhysChem Exam-AnswersDocument10 pages09 (2) PhysChem Exam-Answerstiffanyyy00No ratings yet
- Chemical Reaction Engineering 1 BKF 2453 SEM II 2015/2016: Mini ProjectDocument26 pagesChemical Reaction Engineering 1 BKF 2453 SEM II 2015/2016: Mini ProjectSyarif Wira'iNo ratings yet
- GR 10 - Maths Lit - Step Ahead - Learner SolutionDocument33 pagesGR 10 - Maths Lit - Step Ahead - Learner Solutionkatolicious638No ratings yet
- Mathematical Literacy GR 11 MEMO P1Document5 pagesMathematical Literacy GR 11 MEMO P1w82sm6kw7vNo ratings yet
- Mathematical Literacy GR 10 MEMO Paper 1 1Document3 pagesMathematical Literacy GR 10 MEMO Paper 1 1katolicious638No ratings yet
- M LitDocument16 pagesM LitThato Moratuwa MoloantoaNo ratings yet
- Rate of Chemical Reaction: The Iodination of AcetoneDocument7 pagesRate of Chemical Reaction: The Iodination of AcetoneFaisal MumtazNo ratings yet
- 2021 Chem Skills SolutionsDocument10 pages2021 Chem Skills SolutionsVictor GuanNo ratings yet
- 2015 Assignments With SolutionsDocument48 pages2015 Assignments With Solutionsjesus.mcgiantNo ratings yet
- Ingenieria de La ReaccionesDocument21 pagesIngenieria de La ReaccionesManuela Ospina ArboledaNo ratings yet
- Chemistry 31 - Quantitative Analysis Final Exam, May 18, 2011Document6 pagesChemistry 31 - Quantitative Analysis Final Exam, May 18, 2011Agatha BermudezNo ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Problem 5.31: SolutionDocument3 pagesProblem 5.31: Solutionali ahmedNo ratings yet
- 18s Cpe221 Test1 SolutionDocument4 pages18s Cpe221 Test1 SolutionKyra LathonNo ratings yet
- Ionic EquilibriumDocument55 pagesIonic Equilibriumharshul jainNo ratings yet
- Lab Report 4 Template 2023Document5 pagesLab Report 4 Template 2023conslancio.hkustNo ratings yet
- Chem Principles 7e ISM Focus 07 Even FINALDocument28 pagesChem Principles 7e ISM Focus 07 Even FINALSelma MeloNo ratings yet
- Chem 1110 Midterm Test Winter Term 11Document12 pagesChem 1110 Midterm Test Winter Term 11sanaassaf19No ratings yet
- SMTA1202Document100 pagesSMTA1202natuhari91No ratings yet
- Rates of Reaction Suroviec Spring 2014Document43 pagesRates of Reaction Suroviec Spring 2014enesffsNo ratings yet
- ChemE 2200 Lecture K3Document14 pagesChemE 2200 Lecture K3Bharat Kumar HumagaiNo ratings yet
- Rate Law Worksheet AnswersDocument6 pagesRate Law Worksheet AnswersANGELYN SANTOSNo ratings yet
- CETO2B1Document13 pagesCETO2B1Ontiretse MachailweNo ratings yet
- Reaction Order and Rate Law Expression Worksheet KeyDocument5 pagesReaction Order and Rate Law Expression Worksheet KeyLyra GurimbaoNo ratings yet
- Post-Lab 8 Bleach Redox-ProblemsDocument4 pagesPost-Lab 8 Bleach Redox-ProblemsUzo Paul NwabuisiNo ratings yet
- Chemical Kinetics - Chapter 14Document16 pagesChemical Kinetics - Chapter 14aniedorfNo ratings yet
- JEE Main 2019 Paper Solution Chemistry 09-04-2019 1stDocument9 pagesJEE Main 2019 Paper Solution Chemistry 09-04-2019 1stSaumya KumarNo ratings yet
- Assignment 2-SolutionDocument28 pagesAssignment 2-Solutionleetianyi34No ratings yet
- Universiteti Politeknik I TiranësDocument8 pagesUniversiteti Politeknik I TiranësPC100% (1)
- Chemistry File 4Document4 pagesChemistry File 4Pawan Kumar100% (1)
- Problem 1Document7 pagesProblem 1Alphamae VicenteNo ratings yet
- E-Caps-12 - Class Xii (SS) - Chem - FinalDocument5 pagesE-Caps-12 - Class Xii (SS) - Chem - FinalKrishnendu SahaNo ratings yet
- Chemistry 31 - Quantitative Analysis Exam #1, March 4, 2009Document4 pagesChemistry 31 - Quantitative Analysis Exam #1, March 4, 2009Agatha BermudezNo ratings yet
- Tut4 162039Document8 pagesTut4 162039Mohamed BasheerNo ratings yet
- I. Multiple Choice. Write The Best Answer From The Following ChoicesDocument5 pagesI. Multiple Choice. Write The Best Answer From The Following ChoicesDoom Refuge100% (1)
- Practice Exam 2.4Document6 pagesPractice Exam 2.4jamalNo ratings yet
- Enthalpy Lab #2Document6 pagesEnthalpy Lab #2Riaz JokanNo ratings yet
- How To Solve A Redox Titration Problem - ChemistryDocument6 pagesHow To Solve A Redox Titration Problem - ChemistryAbi AbaNo ratings yet
- Logarithm EngDocument8 pagesLogarithm EngPrashantcool1999No ratings yet
- Chem P2 2016 KcseDocument12 pagesChem P2 2016 KcseombatijuneNo ratings yet
- Fall 2017 - MTH501 - 3 - SOLDocument3 pagesFall 2017 - MTH501 - 3 - SOLbs150202315 Alina AzharNo ratings yet
- IOQP-2020-21 aKAASH Vns RRR - (Answers & Solutions) - Part-2Document9 pagesIOQP-2020-21 aKAASH Vns RRR - (Answers & Solutions) - Part-2Vikash Kr YadavNo ratings yet
- 2507 Physics Paper With Ans MorningDocument4 pages2507 Physics Paper With Ans MorningDr Jaishree SinghNo ratings yet
- Exam2 2004Document17 pagesExam2 2004Hazem AlmasryNo ratings yet
- Rate LawDocument20 pagesRate Lawsarafnawar12345No ratings yet
- Grade 12 Physical Sciences Test 3 26 June 2022Document20 pagesGrade 12 Physical Sciences Test 3 26 June 2022hlayisofilesNo ratings yet
- Equivalent Concept - Titration APSPDocument20 pagesEquivalent Concept - Titration APSPBeena JayNo ratings yet
- Uneb Uace Chemistry Paper 1 2018Document5 pagesUneb Uace Chemistry Paper 1 2018basilkens200061No ratings yet
- Chemistry-6-9-2020 FNDocument12 pagesChemistry-6-9-2020 FNAbhiNo ratings yet
- L10 - Preparing For The Exam PDFDocument17 pagesL10 - Preparing For The Exam PDFedelmandalaNo ratings yet
- Magnesium ConstantDocument15 pagesMagnesium ConstantBenson ShayoNo ratings yet
- Experiment 4 - KINETIC STUDY OF THE REACTION OF KI WITH FeCl3Document4 pagesExperiment 4 - KINETIC STUDY OF THE REACTION OF KI WITH FeCl3Stefani KavangoNo ratings yet
- CHEMICAL KINETIC-worksheet - Module - 2Document3 pagesCHEMICAL KINETIC-worksheet - Module - 2sarahNo ratings yet
- Ans-Sol JEEMain-2022 Phase-2 25-07-2022 M Chemistry FINALDocument7 pagesAns-Sol JEEMain-2022 Phase-2 25-07-2022 M Chemistry FINALChirayu SharmaNo ratings yet
- Climate Change Impact at Three Different Scales of Biological Diversity: A Holistic Review of Research Findings From Across The GlobeDocument7 pagesClimate Change Impact at Three Different Scales of Biological Diversity: A Holistic Review of Research Findings From Across The GlobeIJAR JOURNALNo ratings yet
- Energy Efficiency PPT NotesDocument2 pagesEnergy Efficiency PPT Notesapi-235634024No ratings yet
- Marcus Deon Smith - Letter To Dept of Justice - Pattern and Practice InvestigationDocument6 pagesMarcus Deon Smith - Letter To Dept of Justice - Pattern and Practice InvestigationHashim WarrenNo ratings yet
- Report of One Day Inservice Education - Mamata DasDocument16 pagesReport of One Day Inservice Education - Mamata DasJay Paul100% (1)
- LEEA ACADEMY Course Study Materials - MCE - V1.0 Mar 2020-MergedDocument189 pagesLEEA ACADEMY Course Study Materials - MCE - V1.0 Mar 2020-MergedRana DanishNo ratings yet
- Potential Failure Mode and Effects Analysis (DESIGN FMEA) : Disk Brake Sub-AssemblyDocument6 pagesPotential Failure Mode and Effects Analysis (DESIGN FMEA) : Disk Brake Sub-AssemblyLuis Carlos SuarezNo ratings yet
- Soal UAS01Document8 pagesSoal UAS01Ferdinandus TengaNo ratings yet
- Tourniquet Conversion Drew JSOM Fall 2015 Edition-2Document5 pagesTourniquet Conversion Drew JSOM Fall 2015 Edition-2Oleg ShubinNo ratings yet
- The Goa GuideDocument48 pagesThe Goa GuideNehaNo ratings yet
- 1) Anatomy & Physiology - 202243.15374-CCRN-1505-01 - MANAGEMENT OF CCHC - RESPIRATORYDocument12 pages1) Anatomy & Physiology - 202243.15374-CCRN-1505-01 - MANAGEMENT OF CCHC - RESPIRATORYafshin nikraveshNo ratings yet
- New Promotional & Incentive Structure of PLI & RPLIDocument14 pagesNew Promotional & Incentive Structure of PLI & RPLIkaaushik maityNo ratings yet
- Band III Core Word ListDocument40 pagesBand III Core Word ListKatya RipsNo ratings yet
- API 651 QuestionsDocument4 pagesAPI 651 QuestionsMohammed YoussefNo ratings yet
- Activity 1 - Myths of Aging (Aspuria, Trisha Mae)Document2 pagesActivity 1 - Myths of Aging (Aspuria, Trisha Mae)Trisha Mae AspuriaNo ratings yet
- Cambridge - English 365 Level 1 WordlistDocument5 pagesCambridge - English 365 Level 1 WordlistStrelitzia AugustaNo ratings yet
- Indg 248Document2 pagesIndg 248Ajas AjuNo ratings yet
- Cyto 2.4Document5 pagesCyto 2.4Medtech SoonNo ratings yet
- Jurnal Hair Evaluation Method - DandruffDocument4 pagesJurnal Hair Evaluation Method - DandruffShafiraNo ratings yet
- Nursing Care Plan For A Person With Croup Nursing DiagnosisDocument2 pagesNursing Care Plan For A Person With Croup Nursing DiagnosisMonica Rivera100% (1)
- Risk and Return Thuyết TrìnhDocument16 pagesRisk and Return Thuyết Trìnhdinhduongle2004No ratings yet
- Module 6 RecruitmentDocument14 pagesModule 6 RecruitmentJoyce Ann Aj TomaleNo ratings yet
- Attachment Theory in Adult PsychiatryDocument10 pagesAttachment Theory in Adult PsychiatryEsteli189No ratings yet
- Kitchen Checklist: Where We EatDocument1 pageKitchen Checklist: Where We EatNicoleta AlexandraNo ratings yet
- 2013 Poea Standard Terms and ConditionsDocument8 pages2013 Poea Standard Terms and ConditionsJhon Manuel VelosNo ratings yet
- The Vibrant Nature of Life: Scientific Secrets For Your Journey Through Space and TimeDocument424 pagesThe Vibrant Nature of Life: Scientific Secrets For Your Journey Through Space and TimeDr. Peter Fritz Walter100% (11)
- Komiyama Et Al., (2008) Allometry, Biomass, and Productivity of Mangrove Forests A ReviewDocument11 pagesKomiyama Et Al., (2008) Allometry, Biomass, and Productivity of Mangrove Forests A ReviewVandhi Amali100% (1)
- Oman Rainfall PDFDocument13 pagesOman Rainfall PDFConifer YuNo ratings yet