Professional Documents
Culture Documents
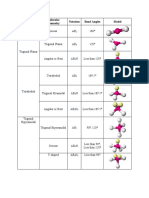
Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles Example
Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles Example
Uploaded by
Richamille Ann Ricaforte0 ratings0% found this document useful (0 votes)
11 views2 pagesThe document outlines the electronic and molecular geometries of molecules based on the number of valence electrons, lone electron pairs, and bonding groups. It shows that as the number of electrons, pairs, and groups changes, the molecular geometry shifts between linear, trigonal, tetrahedral, trigonal bipyramidal, octahedral, and other 3D structures, with bond angles ranging from less than 90 degrees to 180 degrees.
Original Description:
Original Title
chhemmmiiistttrryyy.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document outlines the electronic and molecular geometries of molecules based on the number of valence electrons, lone electron pairs, and bonding groups. It shows that as the number of electrons, pairs, and groups changes, the molecular geometry shifts between linear, trigonal, tetrahedral, trigonal bipyramidal, octahedral, and other 3D structures, with bond angles ranging from less than 90 degrees to 180 degrees.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
11 views2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles Example
Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles Example
Uploaded by
Richamille Ann RicaforteThe document outlines the electronic and molecular geometries of molecules based on the number of valence electrons, lone electron pairs, and bonding groups. It shows that as the number of electrons, pairs, and groups changes, the molecular geometry shifts between linear, trigonal, tetrahedral, trigonal bipyramidal, octahedral, and other 3D structures, with bond angles ranging from less than 90 degrees to 180 degrees.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
Electron Bonding Lone Electronic Molecular Approximate Example
Groups Groups Pairs Geometry Geometry Bond Angles
2 2 0 Linear Linear 180°
3 3 0 Trigonal Trigonal 120°
Planar Planar
3 2 1 Trigonal Bent < 120°
Planar
4 4 0 Tetrahedral Tetrahedral 109.5°
4 3 1 Tetrahedral Trigonal < 109.5°
Pyramidal
4 2 2 Tetrahedral Bent ≪ 109.5°
5 5 0 Trigonal Trigonal 120° (equational)
Bipyramidal Bipyramidal 90° (axial)
5 4 1 Trigonal Seesaw <
Bipyramidal 120° (equational)
< 90° (axial)
5 3 2 Trigonal T-shaped < 90°
Bipyramidal
5 2 3 Trigonal Linear 180°
Bipyramidal
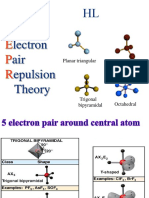
6 6 0 Octahedral Octahedral 90°
6 5 1 Octahedral Square < 90°
Pyramidal
Square
6 4 2 Octahedral Planar 90°
You might also like
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- Molecular GeometryDocument2 pagesMolecular GeometryIsabel SantosNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Electron Domains and Molecular Geometry IBDP ChemistryDocument1 pageElectron Domains and Molecular Geometry IBDP Chemistryyasmeen alkhaterNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- Properties of 2D Shapes - AnswersDocument1 pageProperties of 2D Shapes - Answerscloud scapeNo ratings yet
- Chapter 3 Polygons2Document26 pagesChapter 3 Polygons2Jonard G. TrajanoNo ratings yet
- ACTIVITY SHEET Geometry of Simple CompoundsDocument4 pagesACTIVITY SHEET Geometry of Simple CompoundsUy, Jhavelaine Cassandra F.No ratings yet
- 3 AB Trigonal Planar Trigonal Planar 120 Between All BondsDocument5 pages3 AB Trigonal Planar Trigonal Planar 120 Between All BondsVedantNo ratings yet
- VSEPR GeometriesDocument1 pageVSEPR GeometriesJason JacksonNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Geometry SheetDocument1 pageGeometry Sheetapi-3697114100% (1)
- Unit Circle in Depth HandoutDocument6 pagesUnit Circle in Depth HandoutFayzaNo ratings yet
- TrigonometryDocument31 pagesTrigonometryjifihet931No ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Geometry Revision Summary 4: Angle Types Key VocabularyDocument2 pagesGeometry Revision Summary 4: Angle Types Key VocabularyPol PogbaNo ratings yet
- 6.1 - Radian Measure and Arc Length Math 30-1Document14 pages6.1 - Radian Measure and Arc Length Math 30-1Math 30-1 EDGE Study Guide Workbook - by RTD LearningNo ratings yet
- Actividad Extraclase ADocument2 pagesActividad Extraclase AhubaplaNo ratings yet
- Drawing and Measuring Angles (Answers)Document4 pagesDrawing and Measuring Angles (Answers)pinhaolaurent.valentinaNo ratings yet
- Introduction To MolecularDocument1 pageIntroduction To Molecularclairole quilantangNo ratings yet
- Regular Polygons TableDocument2 pagesRegular Polygons TableGrace HutallaNo ratings yet
- Quarter3 - Module - Week5Document1 pageQuarter3 - Module - Week5JAMAICA PAULA REGALANo ratings yet
- Digital Text BookDocument12 pagesDigital Text BookBindu Vinu100% (1)
- Booklet AnswersDocument1 pageBooklet AnswersEliana UrpianelloNo ratings yet
- Geometría Molecular PDFDocument1 pageGeometría Molecular PDFGeanellaNo ratings yet
- Sol of Angle and Its MeasurementsDocument6 pagesSol of Angle and Its MeasurementsNilesh ShirgireNo ratings yet
- Shapes of Covalent MoleculesDocument5 pagesShapes of Covalent MoleculesSiya ChiniahNo ratings yet
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- AnglesDocument23 pagesAnglespalamaselloNo ratings yet
- Geometry Quick Guide 1: Angles: Angle Types Angle RulesDocument1 pageGeometry Quick Guide 1: Angles: Angle Types Angle RulesROSLINA BINTI ABDUL RASHID MoeNo ratings yet
- Add Maths F5-CHP 1Document13 pagesAdd Maths F5-CHP 1ISMADI SURINNo ratings yet
- UT2 Worksheet - 2 KeyanswersDocument5 pagesUT2 Worksheet - 2 KeyanswersPushpalataNo ratings yet
- Trig+and+PreCalculus+Tutor+ +worksheet+5+ +anglesDocument14 pagesTrig+and+PreCalculus+Tutor+ +worksheet+5+ +angleslefosey723No ratings yet
- F2 C4 Polygons 1Document9 pagesF2 C4 Polygons 1Cally ChewNo ratings yet
- MoleDocument2 pagesMoleapi-233333580No ratings yet
- Trigonometric FunctionsDocument48 pagesTrigonometric Functionsegam0004No ratings yet
- 1 2 PDFDocument2 pages1 2 PDF祁伟No ratings yet
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- Geometry 2 (New)Document27 pagesGeometry 2 (New)Xuan StixxNo ratings yet
- Circle TheoremsDocument18 pagesCircle TheoremsSamuel MalileNo ratings yet
- Measuring Angles in ShapesDocument2 pagesMeasuring Angles in ShapesSimon MakariNo ratings yet
- PRECAL Final Module 1 4Document41 pagesPRECAL Final Module 1 4Glen MillarNo ratings yet
- Pre CalDocument17 pagesPre CalNatalie GaidNo ratings yet
- Precal ModuleeeeeeeDocument18 pagesPrecal ModuleeeeeeeCaila AlfonsoNo ratings yet
- Vsepr ChartDocument1 pageVsepr ChartpankajNo ratings yet
- MathDocument2 pagesMathƦussel ʟobosNo ratings yet
- PRE CALCULUS 2ndQ SLMDocument45 pagesPRE CALCULUS 2ndQ SLMWilmar RonioNo ratings yet
- Grade 10 Chapter 10Document56 pagesGrade 10 Chapter 10Kaung KhantNo ratings yet
- MAT122 - Lesson 1 (2022-2023 Sem 2)Document25 pagesMAT122 - Lesson 1 (2022-2023 Sem 2)Lesley SimonNo ratings yet
- 8-1 Measurement of Arcs (Presentation)Document13 pages8-1 Measurement of Arcs (Presentation)Sandra Miller100% (1)
- Comp8 2ccDocument29 pagesComp8 2ccJoel CaminoNo ratings yet
- Coterminal Angles and Radian MeasureDocument18 pagesCoterminal Angles and Radian MeasureNoli NogaNo ratings yet
- Angle Measure and Unit CircleDocument16 pagesAngle Measure and Unit CircleSnow BollNo ratings yet
- Write Up of All Angle RulesDocument6 pagesWrite Up of All Angle RulesNancy RadwanNo ratings yet
- Chapter 10 TrigonometryDocument35 pagesChapter 10 TrigonometryNay ChiNo ratings yet
- Bab 4 TrigonometryDocument31 pagesBab 4 TrigonometryMuhammad NathanNo ratings yet
- Chapter 11 Triangles, Quadrilaterals and Polygons Exercise 11B 1. (A) ° 90° 54°Document10 pagesChapter 11 Triangles, Quadrilaterals and Polygons Exercise 11B 1. (A) ° 90° 54°johnlimethanNo ratings yet
- MathsTraks: Geometry: A Collection of Blackline Masters for ages 11-14From EverandMathsTraks: Geometry: A Collection of Blackline Masters for ages 11-14No ratings yet