Professional Documents
Culture Documents
Quantum Relationships
Quantum Relationships
Uploaded by
Mary StrangeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quantum Relationships
Quantum Relationships
Uploaded by
Mary StrangeCopyright:
Available Formats
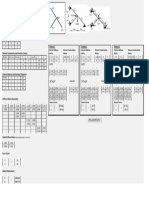
QUANTUM RELATIONSHIPS
Principle Angular Magnetic Quantum Orbital No. of Orbitals Number of
Quantum Momentum Number Name Electrons
Number Quantum Number
n l ml Subshell Shell Subshell Shell
(0, n - 1) (-l … 0 … +l) (2l + 1) (n2) (2n2)
1 0 0 1s 1 1 2 2
2 0 0 2s 1 4 2 8
1 -1, 0, +1 2p 3 6
3 0 0 3s 1 9 2 18
1 -1, 0, +1 3p 3 6
2 -2, -1, 0, +1, +2 3d 5 10
4 0 0 4s 1 16 2 32
1 -1, 0, +1 4p 3 6
2 -2, -1, 0, +1, +2 4d 5 10
3 -3, -2, -1, 0. +1, +2, +3 4f 7 14
SIZE & ENERGY SHAPE ORIENTATION OF
ORBITAL
Nodes: n - l - 1
You might also like
- Starfinder Character Sheet JN10RDocument2 pagesStarfinder Character Sheet JN10RMasterWallwalkerNo ratings yet
- Linear Algebra For Dummies - ErrataDocument8 pagesLinear Algebra For Dummies - ErrataCabecaNo ratings yet
- BY Muhammad Humais Yamin: HomewoekDocument4 pagesBY Muhammad Humais Yamin: HomewoekHumais yaminNo ratings yet
- University of Sialkot: FALL-2020, BS-Chemistry Max. Marks: 10 Course Code: CHEM-3001 Roll No: 18104008-006 Assignment-3Document2 pagesUniversity of Sialkot: FALL-2020, BS-Chemistry Max. Marks: 10 Course Code: CHEM-3001 Roll No: 18104008-006 Assignment-3Samra ButtNo ratings yet
- Sensitifitas DualprimalDocument16 pagesSensitifitas DualprimalCha-cha IchaNo ratings yet
- Qu 2827Document3 pagesQu 2827api-3861295No ratings yet
- 10 - 18040115 - Andre S GintingDocument10 pages10 - 18040115 - Andre S Ginting18-093 Andica Berli HidayatNo ratings yet
- Bending Moment and Shear Force Diagram - Cantilever Both Ends Sse-Excel SectionDocument1 pageBending Moment and Shear Force Diagram - Cantilever Both Ends Sse-Excel SectionSES DESIGNNo ratings yet
- Contoh Soal Di Buku Anstruk: Ndof 2.Nj-Nr 2.4-2 6Document48 pagesContoh Soal Di Buku Anstruk: Ndof 2.Nj-Nr 2.4-2 6Salsabila AshmaliaNo ratings yet
- Stiffness MethodDocument1 pageStiffness MethodasluznetNo ratings yet
- ME320 - Mechanics of Materials Laboratory: TEST TITLE: Combined Stress & Pop Can ExperimentsDocument8 pagesME320 - Mechanics of Materials Laboratory: TEST TITLE: Combined Stress & Pop Can ExperimentsShubhamMaheshwariNo ratings yet
- PDSDocument1 pagePDSRuan encarnaçãoNo ratings yet
- Linear Algebra Homework 5Document9 pagesLinear Algebra Homework 5Bexultan MustafinNo ratings yet
- Numerical Differentiation Problem.Document8 pagesNumerical Differentiation Problem.SKANDA PNo ratings yet
- UntitledDocument3 pagesUntitledwemax979No ratings yet
- Static Moment Sse-Excel Section: The Author Will Not Be Responsible For Any Uses of This Software! v1.06Document1 pageStatic Moment Sse-Excel Section: The Author Will Not Be Responsible For Any Uses of This Software! v1.06SES DESIGNNo ratings yet
- ST LineDocument2 pagesST LineSwaroop MallickNo ratings yet
- Continuum Mechanics and PlasticityDocument2 pagesContinuum Mechanics and PlasticityWilson NaranjoNo ratings yet
- 10.1201 9780203491997-7 PDFDocument2 pages10.1201 9780203491997-7 PDFWilson NaranjoNo ratings yet
- 10.1201 9780203491997-7 PDFDocument2 pages10.1201 9780203491997-7 PDFWilson NaranjoNo ratings yet
- Hasil Pengamatan Acara 1 Dan 2Document8 pagesHasil Pengamatan Acara 1 Dan 2Maya MeisariNo ratings yet
- Goal SeekDocument10 pagesGoal SeekMarko CupacNo ratings yet
- Adobe Scan 14-Mar-2024Document2 pagesAdobe Scan 14-Mar-2024babinarpan436No ratings yet
- PortalDocument3 pagesPortalUmam LufihNo ratings yet
- Examples On Comparison of Survival CurvesDocument3 pagesExamples On Comparison of Survival CurvesKimondo KingNo ratings yet
- Quantum Number ChartDocument1 pageQuantum Number ChartHarry PutrNo ratings yet
- Graf FuncionesDocument4 pagesGraf FuncionesNayly GallardoNo ratings yet
- Ensamble Matriz General 8 X 8Document4 pagesEnsamble Matriz General 8 X 8David AlvarezNo ratings yet
- Método de Las Fuerzas (Formulación Matricial)Document8 pagesMétodo de Las Fuerzas (Formulación Matricial)Guillermo Carranza CiezaNo ratings yet
- Final Exam - Solution 1Document6 pagesFinal Exam - Solution 1ahmedkahlaoui71No ratings yet
- L 04 ExampleDocument20 pagesL 04 ExampleIshit BalarNo ratings yet
- Qu 2826Document2 pagesQu 2826api-3861295No ratings yet
- Problema 1 ADocument2 pagesProblema 1 ASalvador AlvarezNo ratings yet
- Diskusi 5 AljabarDocument2 pagesDiskusi 5 AljabarSDN Kebon Kosong 16 PagiNo ratings yet
- Minimizacion SimplexDocument2 pagesMinimizacion SimplexSergio LuqueNo ratings yet
- PARCIAL I.O Punto 2Document6 pagesPARCIAL I.O Punto 2leonel.quinteroNo ratings yet
- LinearCircuit2 Ch9 SinusoidalDocument34 pagesLinearCircuit2 Ch9 Sinusoidalmakingmunis02No ratings yet
- Atomic OrbitalsDocument63 pagesAtomic Orbitalsmakondo.yhNo ratings yet
- Correlated and Uncorrelated Signals: TransmitDocument34 pagesCorrelated and Uncorrelated Signals: TransmitSangeeta TripathiNo ratings yet
- Return CalculationsDocument13 pagesReturn CalculationshatemNo ratings yet
- Bop Midterm - SolutionsDocument3 pagesBop Midterm - SolutionsAmine BenattouchNo ratings yet
- Kim Loại: Nguyên TỐ Nguyên Tử Khối Hóa Trị Số Oxi HóaDocument2 pagesKim Loại: Nguyên TỐ Nguyên Tử Khối Hóa Trị Số Oxi HóaQuốc Tiên SinhNo ratings yet
- ShapeDocument13 pagesShapePoubelleNo ratings yet
- E 1 I 1 L (M) 6: Barra 1Document6 pagesE 1 I 1 L (M) 6: Barra 1MauricioCaprilesNo ratings yet
- 2A6 Linear Programming - Simplex Method - Minimization Case ILLUSTRATIVE 1st FILEDocument21 pages2A6 Linear Programming - Simplex Method - Minimization Case ILLUSTRATIVE 1st FILERenz ArgarinNo ratings yet
- Resolucion de EjerciciosDocument29 pagesResolucion de EjerciciosAlex Pari FarfanNo ratings yet
- Evaluation and Testing of ResultsDocument10 pagesEvaluation and Testing of ResultsFerdinand IkejiNo ratings yet
- Quay BoxDocument3 pagesQuay BoxYoosu NguyenNo ratings yet
- KarbohidratDocument2 pagesKarbohidratNurna NingsihNo ratings yet
- Full Download Fundamentals of Communication Systems 2nd Edition Proakis Solutions ManualDocument36 pagesFull Download Fundamentals of Communication Systems 2nd Edition Proakis Solutions Manualgiaourgaolbi23a100% (40)
- Metodo de Rigideces: Grados de Libertad Nudo X Y 1 2 3 4Document8 pagesMetodo de Rigideces: Grados de Libertad Nudo X Y 1 2 3 4Miguel Sebastian Valera GarateaNo ratings yet
- Lab #2 Lab TitleDocument10 pagesLab #2 Lab TitleAashutosh PratapNo ratings yet
- Correccion Taller Matematicas 2Document7 pagesCorreccion Taller Matematicas 2angie gonzalezNo ratings yet
- Ejercicio 1 Practica 2 1-2020Document14 pagesEjercicio 1 Practica 2 1-2020BRAYAN MAGNE CACERESNo ratings yet
- Group Activity (Differential Equation)Document8 pagesGroup Activity (Differential Equation)John aubrey MatiasNo ratings yet
- L 7 Simplex Method Max. Numerical M COM IV 16-4-1Document10 pagesL 7 Simplex Method Max. Numerical M COM IV 16-4-1Ashish DeepNo ratings yet
- Calculus 8th Edition Stewart Solutions ManualDocument38 pagesCalculus 8th Edition Stewart Solutions Manualstockeadishureejw100% (17)
- UEM Sol To Exerc Chap 105Document9 pagesUEM Sol To Exerc Chap 105Deola AdebayoNo ratings yet
- SWOT Analysis HW (2015)Document2 pagesSWOT Analysis HW (2015)Mary StrangeNo ratings yet
- Lower Extremity Amputation #8Document7 pagesLower Extremity Amputation #8Mary StrangeNo ratings yet
- HW4A SpineDocument3 pagesHW4A SpineMary StrangeNo ratings yet
- PT616 Comprehensive Capstone Homework 10B/Case Study 10 Pediatrics Team GreenDocument10 pagesPT616 Comprehensive Capstone Homework 10B/Case Study 10 Pediatrics Team GreenMary StrangeNo ratings yet
- Capstone Case Study1 - Quiz 1studyDocument3 pagesCapstone Case Study1 - Quiz 1studyMary StrangeNo ratings yet