Professional Documents
Culture Documents
Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)
Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)
Uploaded by
Shreyash KolekarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)
Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics) (Answers)
Uploaded by
Shreyash KolekarCopyright:
Available Formats
CBSE TEST PAPER-03
CLASS - XII CHEMISTRY (Chemical Kinetics)
[ANSWERS]
Topic: -Determination of order of a reaction
1. a) Since the units of rate constant are Lmol-1 s-1 The reactions is of second order.
b) Since the units of rate constant are s-1 , The reaction is of first order.
2. The rate law will be R = K [NO]2 [H2]
3. R1 = K [A]2 [B] -----------------------------------1)
R2 = K [2A]2 [2B] -----------------------------------2)
Dividing 2) by 1)
R2 [2 A]2 [2B] 8 [A]2 [B] R2
= = = = 8:1 or R 2 = 8R1
R1 [ A]2 [B] [A]2 [B] R1
The rate of reaction increases eight times.
[ R]0 − [ R]
4. i) Zero order reaction - K=
t
2.303 [R ]
ii) First order reaction K= log 0
t [ R]
Where R0 is the initial concentration
R is concentration at time t.
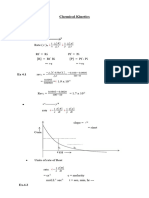
5. From the graph
i) Reaction is first order reaction
ii) The unit of rate constant will be sec-1.
6. Let the order of reaction be x
Rate = K[N2O5]x
i) From the data -
34 ×10-5 = (1.13× 10-2)x ----------------1)
25×10-5 = (0.84× 10-2)x ---------------2)
Material downloaded from http://myCBSEguide.com and http://onlineteachers.co.in
Portal for CBSE Notes, Test Papers, Sample Papers, Tips and Tricks
18×10-5 = (0.62×10-2)x ----------------3)
Dividing 1) by 2)
34 ×10−5 1.13 ×10−2 x
= ( )
25 × 10−5 0.84 ×10−2
(1.36) = (1.35)x
X=1
The order of reaction with respect with respect to N2O5 is 1
ii) Rate law R = K [N2O5]
Rate 18 ×10−5 mol/L/min
iii) Rate constant , K = = = 0.29 min-1
[ N 2O5 ] 0.62 ×10−2 mol/L
7. Order of NO is 2
Rate law = K [Cl2] [NO]2
8.
9. Use of integrated rate equation -
1. The value of rate constant can be known when concentration of reactant at different
times are known-
2. Order of a reaction can be determined by the knowledge of reaction concentration at
different times.
10. For the reaction A → B , if the order =1
− d [ A]
(i) Differential rate law is = K [A]
dt
2.303 [ R]o
(ii) Integrated rate law is t = log
K [ R]
Material downloaded from http://myCBSEguide.com and http://onlineteachers.co.in
Portal for CBSE Notes, Test Papers, Sample Papers, Tips and Tricks
You might also like
- Solutions To Problems, Capitulo 2 LevenspielDocument6 pagesSolutions To Problems, Capitulo 2 LevenspielAlexander Gonzalez Romero75% (4)
- Balancing Chemical Equations Guided Inquiry - StudentHandoutDocument2 pagesBalancing Chemical Equations Guided Inquiry - StudentHandoutamber khasruaNo ratings yet
- Determining The Rate Law For A Reaction Between Iron (III) and Iodide IonDocument4 pagesDetermining The Rate Law For A Reaction Between Iron (III) and Iodide IonValentin-AngeloUzunov100% (12)
- Answer Scheme Practice CORONA - 1-1Document12 pagesAnswer Scheme Practice CORONA - 1-1Mumtaz Barhiya100% (1)
- BT6604 Cre PDFDocument56 pagesBT6604 Cre PDFNIKHIL SHINDENo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Chemical Kinetics)Shreyash KolekarNo ratings yet
- 12 Chemistry Chemical Kinetics Test 03Document2 pages12 Chemistry Chemical Kinetics Test 03Shivanshu SiyanwalNo ratings yet
- 4 BQ - Ans Chemical KineticsDocument7 pages4 BQ - Ans Chemical KineticsDawa PenjorNo ratings yet
- Matriculation Chemistry (Reaction Kinetics) Part 2Document18 pagesMatriculation Chemistry (Reaction Kinetics) Part 2ridwan100% (2)
- Question On Chemical Kinetics-MA 2022Document12 pagesQuestion On Chemical Kinetics-MA 2022Sangay ChodenNo ratings yet
- Lecturers CHEMICAL KINETICSDocument36 pagesLecturers CHEMICAL KINETICSb.hamdi160No ratings yet
- Formula PDFDocument4 pagesFormula PDFMin HwanNo ratings yet
- Review Packet Kinetics AnswersDocument9 pagesReview Packet Kinetics AnswersLama DebanyNo ratings yet
- 5 - 2-Chemical Kinetics (Level)Document33 pages5 - 2-Chemical Kinetics (Level)abdealiarajaNo ratings yet
- Chemical Kinetics and Nuclear ChemistryDocument48 pagesChemical Kinetics and Nuclear ChemistryAnurag KasaudhanNo ratings yet
- Chemical Kinetic PDFDocument44 pagesChemical Kinetic PDFAmogh VaishnavNo ratings yet
- ObjectivesDocument24 pagesObjectivesAvicenna Ibnu BahrinNo ratings yet
- 1.0 Reaction KineticsDocument142 pages1.0 Reaction KineticsKhairul Aswari Ab RahmanNo ratings yet
- Matriculation Chemistry (Reaction Kinetics) Part 2Document33 pagesMatriculation Chemistry (Reaction Kinetics) Part 2ridwanNo ratings yet
- Chemical Kinetics - 2Document20 pagesChemical Kinetics - 2bhawnam.1995No ratings yet
- 102 MSJC 13Document11 pages102 MSJC 13noelNo ratings yet
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaDocument34 pagesChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaAngelNo ratings yet
- Test On Chemical KineticsDocument4 pagesTest On Chemical Kineticsdevansh dewanNo ratings yet
- Chemical Kinetics: Rate of Reaction MechanismDocument60 pagesChemical Kinetics: Rate of Reaction MechanismAneudis Javier BritoNo ratings yet
- 1 Kinetics Jee Main Based Test With AnsDocument7 pages1 Kinetics Jee Main Based Test With AnsquizsanswersNo ratings yet
- 1.0 Kinetics 2020 - 2021 (Lecturer)Document15 pages1.0 Kinetics 2020 - 2021 (Lecturer)siti aisyahNo ratings yet
- Chemical Kinetics: General Chemistry 2Document27 pagesChemical Kinetics: General Chemistry 2France TurdaNo ratings yet
- Chemical KineticsDocument7 pagesChemical Kineticsthinkiit100% (1)
- 11.1 Reaction Rate 2Document17 pages11.1 Reaction Rate 2Avicenna Ibnu Bahrin100% (1)
- Log K ®: Section A //X Choose Correct Answer From The Given Options. (Each Carries 1 Mark)Document20 pagesLog K ®: Section A //X Choose Correct Answer From The Given Options. (Each Carries 1 Mark)WhoaretoNo ratings yet
- Neet-Jee KineticsDocument16 pagesNeet-Jee KineticsSudheerkhan MuhammedNo ratings yet
- Chapter 14Document95 pagesChapter 14Tanisha SenNo ratings yet
- Chemical KineticsDocument18 pagesChemical KineticsRamchandra MurthyNo ratings yet
- Chem101103 Summerfinalexam SolutionDocument5 pagesChem101103 Summerfinalexam SolutionbrianNo ratings yet
- Questions - Answers Bank Class - Xii Subject - Chemistry UNIT-4 (Chemical Kinetics)Document3 pagesQuestions - Answers Bank Class - Xii Subject - Chemistry UNIT-4 (Chemical Kinetics)Abhay BharadwajNo ratings yet
- Kinetika KimiaDocument39 pagesKinetika KimiaMia YukimuraNo ratings yet
- M/s. What Is The Rate of Change of A?Document3 pagesM/s. What Is The Rate of Change of A?vikyappleNo ratings yet
- CLS ENG 23 24 XII Che Target 2 Level 1 Chapter 4Document54 pagesCLS ENG 23 24 XII Che Target 2 Level 1 Chapter 4mohitverma2133No ratings yet
- A K T A X: 4. Chemical KineticsDocument4 pagesA K T A X: 4. Chemical KineticseamcetmaterialsNo ratings yet
- 11 Reaction KineticsDocument95 pages11 Reaction KineticsSyamil Adzman100% (1)
- Chapter 4Document19 pagesChapter 4Devansh GangwarNo ratings yet
- Kinetics Lec-1 NEET ChalisaDocument35 pagesKinetics Lec-1 NEET Chalisaashustarguy005No ratings yet
- Lecture Notes Module 5 KineticsDocument12 pagesLecture Notes Module 5 KineticsFaith SmithNo ratings yet
- Chem 312 Test 1 2013 MemoDocument11 pagesChem 312 Test 1 2013 Memomatloa71No ratings yet
- Model Solution 1Document6 pagesModel Solution 1Rts SNo ratings yet
- Lecture 14 Slides Kinetics Week 5-2 PDFDocument34 pagesLecture 14 Slides Kinetics Week 5-2 PDFSam YuritrevNo ratings yet
- CBSE NCERT Solutions For Class 12 Chemistry Chapter 4: Back of Chapter QuestionsDocument33 pagesCBSE NCERT Solutions For Class 12 Chemistry Chapter 4: Back of Chapter QuestionsVijay Pratap Singh RathoreNo ratings yet
- Chemistry Chemical KineticsDocument4 pagesChemistry Chemical KineticsSurya PrakashNo ratings yet
- Part - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Chemical KineticsDocument38 pagesPart - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Chemical KineticsdfaNo ratings yet
- Chemistry File 4Document4 pagesChemistry File 4Pawan Kumar100% (1)
- Ashma Ma'Am-chemical Kinetics 2Document7 pagesAshma Ma'Am-chemical Kinetics 2Joyce PereiraNo ratings yet
- Hsslive-4. CHEMICAL KINETICS Previous HSE Qns. With AnswersDocument9 pagesHsslive-4. CHEMICAL KINETICS Previous HSE Qns. With Answerssindhumv631No ratings yet
- Chem1b E1 PracDocument24 pagesChem1b E1 PracBurt NguyenNo ratings yet
- Pyqa-Xii-Che-4. Chemical KineticsDocument11 pagesPyqa-Xii-Che-4. Chemical Kineticsnidha8225No ratings yet
- Chemical Kinetics AssinmentDocument9 pagesChemical Kinetics AssinmentKhushi TiwariNo ratings yet
- Kinetika Kimia s1Document28 pagesKinetika Kimia s1Umiia RuhunussaNo ratings yet
- CHEMICAL KINETIC-worksheet - Module - 2Document3 pagesCHEMICAL KINETIC-worksheet - Module - 2sarahNo ratings yet
- AK - 4 - Chemical KineticsDocument8 pagesAK - 4 - Chemical Kineticsmgupta13marNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsShivani VermaNo ratings yet
- Bakliwal Tutorials - IIT: ChemistryDocument1 pageBakliwal Tutorials - IIT: ChemistrySACHIN KASERANo ratings yet
- Rate LawsDocument19 pagesRate LawsEli BerkowitzNo ratings yet
- Trigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsFrom EverandTrigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsRating: 5 out of 5 stars5/5 (1)
- Inverse Trigonometric Functions (Trigonometry) Mathematics Question BankFrom EverandInverse Trigonometric Functions (Trigonometry) Mathematics Question BankNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) (Answers)Document3 pagesCbse Test Paper-02 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) (Answers)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) (Answer)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) (Answer)Shreyash KolekarNo ratings yet
- Cbse Test Paper-04 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Document2 pagesCbse Test Paper-04 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Shreyash KolekarNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Document2 pagesCbse Test Paper-02 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Document1 pageCbse Test Paper-03 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Shreyash KolekarNo ratings yet
- CHCHCHBR X Y Z: Cbse Test Paper-04 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Document1 pageCHCHCHBR X Y Z: Cbse Test Paper-04 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes)Shreyash KolekarNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements)Document1 pageCbse Test Paper-03 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Document1 pageCbse Test Paper-03 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements) (Answers)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer) Topic: - F-Block Elements: Lanthanoids and ActinoidsDocument1 pageCbse Test Paper-03 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer) Topic: - F-Block Elements: Lanthanoids and ActinoidsShreyash KolekarNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements)Document1 pageCbse Test Paper-02 CLASS - XII CHEMISTRY (General Principles and Processes of Isolation of Elements)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Coordination Compounds) (Answer)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Coordination Compounds) (Answer)Shreyash KolekarNo ratings yet
- 12 Chemistry Haloalkanes and Haloarenes Test 05 Answer s2l6 PDFDocument2 pages12 Chemistry Haloalkanes and Haloarenes Test 05 Answer s2l6 PDFShreyash KolekarNo ratings yet
- 12 Chemistry Coordination Compounds Test 03 PDFDocument1 page12 Chemistry Coordination Compounds Test 03 PDFShreyash KolekarNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer)Shreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Document1 pageCbse Test Paper-03 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Shreyash KolekarNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Coordination Compounds) (Answer)Document2 pagesCbse Test Paper-01 CLASS - XII CHEMISTRY (Coordination Compounds) (Answer)Shreyash KolekarNo ratings yet
- Cbse Test Paper-04 CLASS - XII CHEMISTRY (Coordination Compounds)Document3 pagesCbse Test Paper-04 CLASS - XII CHEMISTRY (Coordination Compounds)Shreyash KolekarNo ratings yet
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Document1 pageCbse Test Paper-01 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Shreyash KolekarNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Document1 pageCbse Test Paper-02 CLASS - XII CHEMISTRY (The D - & F-Block Elements)Shreyash KolekarNo ratings yet
- Cbse Test Paper-04 CLASS - XII CHEMISTRY (Coordination Compounds)Document1 pageCbse Test Paper-04 CLASS - XII CHEMISTRY (Coordination Compounds)Shreyash KolekarNo ratings yet
- Cbse Test Paper-05 CLASS - XII CHEMISTRY (Coordination Compounds)Document2 pagesCbse Test Paper-05 CLASS - XII CHEMISTRY (Coordination Compounds)Shreyash KolekarNo ratings yet
- 12 Chemistry Electrochemistry Test 01 Answer 8b9m PDFDocument2 pages12 Chemistry Electrochemistry Test 01 Answer 8b9m PDFShreyash KolekarNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer)Document2 pagesCbse Test Paper-02 CLASS - XII CHEMISTRY (The D - & F-Block Elements) (Answer)Shreyash KolekarNo ratings yet
- Cbse Test Paper-02 CLASS - XII CHEMISTRY (Electrochemistry) (Answers)Document3 pagesCbse Test Paper-02 CLASS - XII CHEMISTRY (Electrochemistry) (Answers)Shreyash KolekarNo ratings yet
- 12 Chemistry Electrochemistry Test 02 PDFDocument2 pages12 Chemistry Electrochemistry Test 02 PDFShreyash KolekarNo ratings yet
- 12 Chemistry Electrochemistry Test 03 PDFDocument1 page12 Chemistry Electrochemistry Test 03 PDFShreyash KolekarNo ratings yet
- Cbse Test Paper-03 CLASS - XII CHEMISTRY (Electrochemistry) (Answers)Document2 pagesCbse Test Paper-03 CLASS - XII CHEMISTRY (Electrochemistry) (Answers)Shreyash KolekarNo ratings yet
- 12 Chemistry Electrochemistry Test 04 PDFDocument1 page12 Chemistry Electrochemistry Test 04 PDFShreyash KolekarNo ratings yet
- 12 Chemistry Electrochemistry Test 05 PDFDocument1 page12 Chemistry Electrochemistry Test 05 PDFShreyash KolekarNo ratings yet
- Catalase Bio LabDocument3 pagesCatalase Bio LabDaVejar42No ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsSamarNo ratings yet
- Cumene AdsorptionDocument33 pagesCumene AdsorptionAasim ShaikhNo ratings yet
- Chapter 1 PDFDocument74 pagesChapter 1 PDFLi Tan100% (1)
- Angew Chem Int Ed - 2015 - Pan - Total Synthesis of Diterpenoid Steenkrotin ADocument4 pagesAngew Chem Int Ed - 2015 - Pan - Total Synthesis of Diterpenoid Steenkrotin Azhang quanNo ratings yet
- The Bromination of Acetone Lab ReportDocument4 pagesThe Bromination of Acetone Lab ReportSammy Njenga KhanNo ratings yet
- Week 4 - Theory - Isothermal Reactor Design Steady StateDocument39 pagesWeek 4 - Theory - Isothermal Reactor Design Steady StateJules ArseneNo ratings yet
- Smith6e Chapter24 TBDocument18 pagesSmith6e Chapter24 TBandrew.gregory978No ratings yet
- Chapter 4 Organic Chemisty WadeDocument44 pagesChapter 4 Organic Chemisty WadeJanet N MarinovaNo ratings yet
- Organic: MechanismsDocument14 pagesOrganic: Mechanismsiswaryas1409No ratings yet
- Thermodynamic Versus Kinetic Reaction ControlDocument15 pagesThermodynamic Versus Kinetic Reaction ControlUdasi Raqs Kerti HaiNo ratings yet
- Reaction Kinetics and Scale-Up of Catalytic ProcessesDocument6 pagesReaction Kinetics and Scale-Up of Catalytic ProcessessatishchemengNo ratings yet
- 4MBBS101 Lecture 5 Properties of Enzymes - Enzyme KineticsDocument51 pages4MBBS101 Lecture 5 Properties of Enzymes - Enzyme KineticsArm UdomratNo ratings yet
- Chemical Reactions ReviewerDocument16 pagesChemical Reactions Reviewerche cheNo ratings yet
- Balancing Redox Reactions WorksheetDocument2 pagesBalancing Redox Reactions Worksheetsai sreerama mNo ratings yet
- DPP 02Document1 pageDPP 02prathmfedNo ratings yet
- Collision TheoryDocument14 pagesCollision TheoryAkshatKhannaNo ratings yet
- Handbook of Organopalladium Chemistry For Organic Synthesis Vol 1 - Negishi-0041 - 42Document3 pagesHandbook of Organopalladium Chemistry For Organic Synthesis Vol 1 - Negishi-0041 - 42gombossandor0% (1)
- Nucleophilic SubstitutionDocument18 pagesNucleophilic SubstitutionShivam GuptaNo ratings yet
- Chemical KineticsDocument8 pagesChemical KineticsSnehashis BoseNo ratings yet
- Lms Template - Tutorial Chap 5Document4 pagesLms Template - Tutorial Chap 5LeticiaNo ratings yet
- Kinetic Models For Nonelementary Reactions: Non Chain ReactionDocument6 pagesKinetic Models For Nonelementary Reactions: Non Chain ReactionXxxNo ratings yet
- Chapter 22. Carbonyl Alpha-Substitution Reactions: Based On Mcmurry'S Organic Chemistry, 9 EditionDocument64 pagesChapter 22. Carbonyl Alpha-Substitution Reactions: Based On Mcmurry'S Organic Chemistry, 9 Edition張湧浩No ratings yet
- SN1, SN2, E1, E2Document39 pagesSN1, SN2, E1, E2Dian AnggrainiNo ratings yet
- Ilovepdf Merged 3Document104 pagesIlovepdf Merged 3api-533764142No ratings yet
- AS Biology BYB1 Core Principles 10.4 Enzymes TestDocument5 pagesAS Biology BYB1 Core Principles 10.4 Enzymes TestThao TrinhNo ratings yet