Professional Documents
Culture Documents
Mux100h Ce PDF
Mux100h Ce PDF
Uploaded by
Niwrad Montilla0 ratings0% found this document useful (0 votes)
66 views1 pageOriginal Title
MUX100H_CE.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
66 views1 pageMux100h Ce PDF
Mux100h Ce PDF
Uploaded by
Niwrad MontillaCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 1
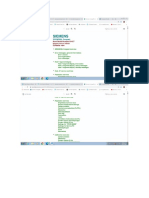
E) SHIMADZU
Declaration of conformity
according to Medical Device Directive 93/42/EEC
Manufacturer = SHIMADZU CORPORATION
‘Medical Systems Division
Address : 1, NISHINOKYO-KUWABARACHO,
NAKAGYO-KU,KYOTO,604-8511,JAPAN
declares, in sole responsibility, that the following product
Product name : Mobile X-ray Unit
ModelName + ‘MUX-100H
Parte Number : 503-48500-12
Which i compliance with Annes I for 93/42/EEC
and which are certified for EN 60601-I,-1-3,-1-4,-2-7,-2-28,-2-32
standard by TOV Rhelnland as Certificate No. DE 2-007339
Also, this product is certified for Geprifters Medizinprodukt (GM)
by TOV Rheinland Product Safety GmbH as Certification No. 150058357.
‘The company’s Quality System is satisfied with Annex Il, Article 3 for 93/42/BEC,
which is certified by TOV Rheinland Product Safety GmbH (Nortified under No. 0197)
as Registration No.: HD 60011592 0001
‘The company named above will keep on file for review the following technical documentation:
*operating and maintenance instructions
‘technical drawings
* description of measures designed to measure conformity
“other technical documentation, eg. quality assurance measures for design and production
Importer/Distributor and Authorized Representative in EU SHIMADZU Deutschland GmbH
Address “Albert-Hahn-Strasse 6-10,D-47269
Duisuburg, F.R.Germany
Note: This declaration becomes invalid if technical or operational modifications are introduced without the
manufacturer's consent.
Iy.2005 issued date) Akins Ahiszyoone (gous)
Akita Shit (full name)
General Manager, Quality Assurance Dept
Medical Systems Div.
Shimadzu Comporation ___ (position)
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Service Manual BV Family SMCM BV R1.1Document112 pagesService Manual BV Family SMCM BV R1.1Niwrad Montilla100% (10)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Kp100 Panel Lust ManualDocument45 pagesKp100 Panel Lust ManualNiwrad MontillaNo ratings yet
- BV300 Service Manuals (Volume 1 and 2)Document1,100 pagesBV300 Service Manuals (Volume 1 and 2)Niwrad MontillaNo ratings yet
- Philips BV-25 Digistore 1 - Service ManualDocument78 pagesPhilips BV-25 Digistore 1 - Service ManualNiwrad MontillaNo ratings yet
- Cargador bv300Document26 pagesCargador bv300Niwrad MontillaNo ratings yet
- Siemens Compact Codigo de ErroresDocument148 pagesSiemens Compact Codigo de ErroresNiwrad Montilla100% (1)
- CR-DR Remote and Key Operator Software Upgrade Instructions PDFDocument74 pagesCR-DR Remote and Key Operator Software Upgrade Instructions PDFNiwrad MontillaNo ratings yet
- Preinstalacion Tomografo PDFDocument5 pagesPreinstalacion Tomografo PDFNiwrad MontillaNo ratings yet
- Connection Diagram MUX100HDocument57 pagesConnection Diagram MUX100HNiwrad MontillaNo ratings yet
- MUX100H Part M5034008ADocument148 pagesMUX100H Part M5034008ANiwrad MontillaNo ratings yet