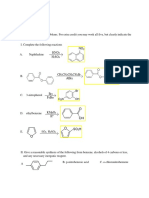
Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
25 viewsIn2Rpt (7) Formate
In2Rpt (7) Formate
Uploaded by
Nosayba M AlomariThis document describes an inorganic chemistry laboratory experiment on the preparation of two transition metal complexes - tris(acetylacetonato)iron(III) ([Fe(acac)3]) and bis(acetylacetonato)diaquanickel(II) ([Ni(acac)2(H2O)2]). The experiment involves reacting iron(III) chloride hexahydrate with acetylacetone to produce [Fe(acac)3], and reacting nickel(II) sulfate hexahydrate with acetylacetone to produce [Ni(acac)2(H2O)2]. Percentage yields are calculated for both complexes. Students are asked to record observations, balanced equations,
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Example Form 1 Science Paper (With Answers)Document10 pagesExample Form 1 Science Paper (With Answers)Kenny Cheah Soon Lee100% (8)
- Experiment 8 The Preparation of AcetanlideDocument12 pagesExperiment 8 The Preparation of AcetanlideRadhwanNo ratings yet
- Wet Scrubber BulletinDocument8 pagesWet Scrubber BulletinHESuarez100% (1)
- Preparation of BakeliteDocument8 pagesPreparation of Bakeliteusman_uet0868% (19)
- Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International General Certificate of Secondary EducationYasser ZubaidiNo ratings yet
- Chemistry 5070 End of YearDocument7 pagesChemistry 5070 End of Yearsamuelbandamiracle20No ratings yet
- Singapore-Cambridge GCE A Levels Exam Practice Paper IIDocument5 pagesSingapore-Cambridge GCE A Levels Exam Practice Paper IIChong56No ratings yet
- Paper 2 Final (Qs Only)Document17 pagesPaper 2 Final (Qs Only)chuasioklengNo ratings yet
- Bengkel Ambang SPM 2009 Kertas 2Document31 pagesBengkel Ambang SPM 2009 Kertas 2azharsarahNo ratings yet
- Chemistry Form 4 Paper 2Document12 pagesChemistry Form 4 Paper 2nuhrramah2912No ratings yet
- Blio 2Document6 pagesBlio 2karenNo ratings yet
- FY 425 (Chemistry)Document12 pagesFY 425 (Chemistry)Shahzad TNo ratings yet
- CHEMISTRY-P2-Teacher Co KeDocument8 pagesCHEMISTRY-P2-Teacher Co KeASSIGNMENTS PRIORITYNo ratings yet
- IGCSE Chemistry Stoichiometry Worksheet-Empirical FormulaDocument5 pagesIGCSE Chemistry Stoichiometry Worksheet-Empirical Formulaabd rahmanNo ratings yet
- CHAPTER 9 Electrochemistry Structure and Essay 13-19Document5 pagesCHAPTER 9 Electrochemistry Structure and Essay 13-19peter edwardNo ratings yet
- Chem 2Document12 pagesChem 2githukucharles.gcNo ratings yet
- Chemistry Pre-Board STD Sem 2 X Gokuldham High School 2021-22Document8 pagesChemistry Pre-Board STD Sem 2 X Gokuldham High School 2021-22Lmao XdNo ratings yet
- HKALE Mock Paper II (2010)Document14 pagesHKALE Mock Paper II (2010)tiffany5No ratings yet
- 962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)Document12 pages962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)sherry_christyNo ratings yet
- Questions & Answers: For For For For For NEET (UG) - 2020Document21 pagesQuestions & Answers: For For For For For NEET (UG) - 2020Ritu JoharNo ratings yet
- 8-Io Chemistry Paper IIDocument3 pages8-Io Chemistry Paper IIleo3ariNo ratings yet
- Section B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)Document8 pagesSection B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)harrynghomanNo ratings yet
- STPM Trial Negeri Sembilan 2007 Chemistry Paper 2Document21 pagesSTPM Trial Negeri Sembilan 2007 Chemistry Paper 2stuart5051No ratings yet
- Chemistry Paper 2 Sabah STPM 2008 Excel Set 2 (Edu - Joshuatly.com)Document13 pagesChemistry Paper 2 Sabah STPM 2008 Excel Set 2 (Edu - Joshuatly.com)HaRry ChgNo ratings yet
- Chemistry Paper 2 Exam PremiDocument12 pagesChemistry Paper 2 Exam PremiSakinah Saad100% (3)
- Chemistry Paper 1: All Questions Are CompulsoryDocument8 pagesChemistry Paper 1: All Questions Are CompulsoryBhupesh Gupta100% (1)
- Energy Changes Revision ModuleDocument7 pagesEnergy Changes Revision Modulegabrielsuva6No ratings yet
- Chemistry Question PaperDocument4 pagesChemistry Question Paperdrsayan09No ratings yet
- MSS 1718MockPaper2Document8 pagesMSS 1718MockPaper2Kelvin ChowNo ratings yet
- Bengkel Ambang SPM 2009paper 2Document31 pagesBengkel Ambang SPM 2009paper 2Mimi MaliniNo ratings yet
- C Ch-18 Co-Ordination CompoundsDocument7 pagesC Ch-18 Co-Ordination Compoundsmysoftinfo.incNo ratings yet
- F3 Scie ExamDocument6 pagesF3 Scie Examhmatara8No ratings yet
- EstherDocument26 pagesEstherEnock SemweziNo ratings yet
- STPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)Document13 pagesSTPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)kokpin100100% (1)
- CHEMISTRY 2 - Questions N AnswersDocument32 pagesCHEMISTRY 2 - Questions N Answersjuliuskamande844No ratings yet
- Chemistry Form 1 Assignment April 2021 Teacher - Co - .KeDocument5 pagesChemistry Form 1 Assignment April 2021 Teacher - Co - .Kejkipyegon175No ratings yet
- Kibugo - Set ThreeDocument6 pagesKibugo - Set ThreeTalemwa ALFRED KAKORAKINo ratings yet
- HKDSE Chemistry: (Paper 2) Mock Examination 4Document6 pagesHKDSE Chemistry: (Paper 2) Mock Examination 4Vinaigrette HeNo ratings yet
- Chem Form 2 End Term 3Document8 pagesChem Form 2 End Term 3DenisNo ratings yet
- ChemDocument19 pagesChemrussell_mahmoodNo ratings yet
- ch212 Ex5Document6 pagesch212 Ex5nkwaneleNo ratings yet
- Chem pp2Document9 pagesChem pp2ewawireNo ratings yet
- Chemistry Paper 4 October 2004Document11 pagesChemistry Paper 4 October 2004Dean DambazaNo ratings yet
- Chemistry Semester 2 SpecimensDocument6 pagesChemistry Semester 2 SpecimensPB electronicsNo ratings yet
- Chemistry Paper 2 Exam PremiDocument12 pagesChemistry Paper 2 Exam PremiEmily VinciNo ratings yet
- A Level Chemistry Paper 2 Exam 8Document5 pagesA Level Chemistry Paper 2 Exam 8Anthony AndyNo ratings yet
- 2000-2019 Nesa Chemistry Advanced Level-1Document269 pages2000-2019 Nesa Chemistry Advanced Level-1Jeff AlbaNo ratings yet
- Chrmistry Form 4 Chapter 3 Chemical Formulae and EquationsDocument8 pagesChrmistry Form 4 Chapter 3 Chemical Formulae and EquationsEric Wong0% (1)
- Time: 3.00 Hours) : This Question Paper Contains 8 Printed PagesDocument8 pagesTime: 3.00 Hours) : This Question Paper Contains 8 Printed PagesrafikdmeNo ratings yet
- Answer Module 11A-Manufactured Substances in IndustryDocument7 pagesAnswer Module 11A-Manufactured Substances in IndustryYen ZyNo ratings yet
- Chempage - 2022 Chemistry Mock Exam 2022 - Chem - Mock - 2 - QBDocument10 pagesChempage - 2022 Chemistry Mock Exam 2022 - Chem - Mock - 2 - QBChun Kit LauNo ratings yet
- Paper 3 SPM 2011 Mastery PracticesDocument30 pagesPaper 3 SPM 2011 Mastery PracticesaganbasmNo ratings yet
- SK025 KMJ Pre PSPM Set 4 (Question)Document4 pagesSK025 KMJ Pre PSPM Set 4 (Question)2022674978No ratings yet
- f4 CHM Pp2 Et1 Qns Teacher Co KeDocument10 pagesf4 CHM Pp2 Et1 Qns Teacher Co KeRedemptaNo ratings yet
- Chem F4 Mid ExamDocument10 pagesChem F4 Mid ExamYong SiewkuanNo ratings yet
- BECO UACE Chem2Document6 pagesBECO UACE Chem2EMMANUEL BIRUNGINo ratings yet
- Semester 2 Examination CHEMISTRY - Mock Paper (Science Paper 2)Document8 pagesSemester 2 Examination CHEMISTRY - Mock Paper (Science Paper 2)Harshith GowdaNo ratings yet
- Specimen Paper 4Document26 pagesSpecimen Paper 4Thanusha DhanarajNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Experiment No. 2Document6 pagesExperiment No. 2mayankroy9431No ratings yet
- The Mole and Stoichiometry: Multiple Choice QuestionsDocument59 pagesThe Mole and Stoichiometry: Multiple Choice QuestionsRIKI MUHAMMADNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 6Document11 pagesRTS Chemistry SPM Question Bank Chapter 6Vincent Vetter100% (1)
- Carbon and Its CompoundsDocument8 pagesCarbon and Its Compoundsbhumika motiyaniNo ratings yet
- HSC 2016 March ChemistryDocument3 pagesHSC 2016 March ChemistryYSDNo ratings yet
- STPM 2013 Sem 2Document5 pagesSTPM 2013 Sem 2m-4306022No ratings yet
- SBS006 Antibacterial Hand CleanerDocument1 pageSBS006 Antibacterial Hand CleanerFloraNo ratings yet
- 2021 H2 Chemistry Prelim Paper 2Document24 pages2021 H2 Chemistry Prelim Paper 2clarissa yeoNo ratings yet
- Ken338 TDocument13 pagesKen338 TAri DanteNo ratings yet
- CHM 212 Lecture NotesDocument14 pagesCHM 212 Lecture Notesdaniel mwantiNo ratings yet
- 11 Chemistry Solved 01 NewDocument4 pages11 Chemistry Solved 01 NewasdfghjklNo ratings yet
- Cold Process Baking Soda Soap Recipe: MaterialsDocument2 pagesCold Process Baking Soda Soap Recipe: MaterialsMaria José PradoNo ratings yet
- Chemistry Calculations: Type of Calculation Revised?Document100 pagesChemistry Calculations: Type of Calculation Revised?Foxy world 152No ratings yet
- Modified-Qualitative Analysis-QuestionDocument5 pagesModified-Qualitative Analysis-QuestionHimanshu GusainNo ratings yet
- Asakawa 1978Document2 pagesAsakawa 1978Omer MukhtarNo ratings yet
- DENSIDADES DE MASAS DE PARTICULAS. Chap21. PERRYÂ SDocument1 pageDENSIDADES DE MASAS DE PARTICULAS. Chap21. PERRYÂ SStefany Mariela Pineda AyalaNo ratings yet
- Lipids Part 3Document9 pagesLipids Part 3Anonymous 596wAK78eCNo ratings yet
- EDEXCEL A2 CHEMISTRY UNIT 5 January 2011Document24 pagesEDEXCEL A2 CHEMISTRY UNIT 5 January 2011Ghaleb W. MihyarNo ratings yet
- Chemicals Specification ManualDocument139 pagesChemicals Specification Manualvelu.gNo ratings yet
- Redox ReactionDocument3 pagesRedox ReactionPranjal GuptaNo ratings yet
- Ring IndexDocument194 pagesRing IndexlalitarunNo ratings yet
- Chemistry-Orgo II Exam 1 Version A (UD) Answer KeyDocument8 pagesChemistry-Orgo II Exam 1 Version A (UD) Answer KeyNesrine LaradjiNo ratings yet
- Experiment No. 5 Preparation of Aspirin (Initial)Document2 pagesExperiment No. 5 Preparation of Aspirin (Initial)Christine MarcellanaNo ratings yet
- Example of Laboratory ReportDocument5 pagesExample of Laboratory Reportpowasloopas258No ratings yet
- Bleaching 1643433388703Document7 pagesBleaching 1643433388703Ishaan GuptaNo ratings yet
- Macromolecules (Polymers, Carbohydrates, Proteins, and Fats)Document27 pagesMacromolecules (Polymers, Carbohydrates, Proteins, and Fats)Melva SibaraniNo ratings yet
- Mixed Aldol CondensationDocument3 pagesMixed Aldol CondensationSangeeta BansalNo ratings yet
In2Rpt (7) Formate
In2Rpt (7) Formate
Uploaded by
Nosayba M Alomari0 ratings0% found this document useful (0 votes)
25 views3 pagesThis document describes an inorganic chemistry laboratory experiment on the preparation of two transition metal complexes - tris(acetylacetonato)iron(III) ([Fe(acac)3]) and bis(acetylacetonato)diaquanickel(II) ([Ni(acac)2(H2O)2]). The experiment involves reacting iron(III) chloride hexahydrate with acetylacetone to produce [Fe(acac)3], and reacting nickel(II) sulfate hexahydrate with acetylacetone to produce [Ni(acac)2(H2O)2]. Percentage yields are calculated for both complexes. Students are asked to record observations, balanced equations,
Original Description:
inorganic laboratory sheet
Original Title
In2Rpt[7]formate
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document describes an inorganic chemistry laboratory experiment on the preparation of two transition metal complexes - tris(acetylacetonato)iron(III) ([Fe(acac)3]) and bis(acetylacetonato)diaquanickel(II) ([Ni(acac)2(H2O)2]). The experiment involves reacting iron(III) chloride hexahydrate with acetylacetone to produce [Fe(acac)3], and reacting nickel(II) sulfate hexahydrate with acetylacetone to produce [Ni(acac)2(H2O)2]. Percentage yields are calculated for both complexes. Students are asked to record observations, balanced equations,
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
25 views3 pagesIn2Rpt (7) Formate
In2Rpt (7) Formate
Uploaded by
Nosayba M AlomariThis document describes an inorganic chemistry laboratory experiment on the preparation of two transition metal complexes - tris(acetylacetonato)iron(III) ([Fe(acac)3]) and bis(acetylacetonato)diaquanickel(II) ([Ni(acac)2(H2O)2]). The experiment involves reacting iron(III) chloride hexahydrate with acetylacetone to produce [Fe(acac)3], and reacting nickel(II) sulfate hexahydrate with acetylacetone to produce [Ni(acac)2(H2O)2]. Percentage yields are calculated for both complexes. Students are asked to record observations, balanced equations,
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 3
Exp.(7) Preparation of [Fe(acac)3] & Ni(acac)2(H2O)2] Chem.
323
Inorganic Chemistry Laboratory (II)
Experiment Number 7
Preparation of Tris(acetylacetonato)iron (III); [Fe(acac)3]
Preparation of Bis(acetylacetonato)diaquanickel (II); [Ni(acac)2(H2O)2]
_____________________________________________________________________________________________________________________
Student Name: ………………………..………… Section: ……………………………….……………
Date of Experiment: …………………….……… Date of Submission: ……………………….…..
NOTE: Late submission of your report lab will be met with a 10-point deduction for every calendar day it is late.
A. Preparation of Tris(acetylacetonato)iron (III); [Fe(acac)3]
Write the balanced equation of reaction:
Color of yield
Weight of [Fe(acac)3] ( actual yield (g))
Number of moles of FeCl3.6H2O
Number of moles of acetylacetone.
The Limiting reagent
Number of moles of [Fe(acac)3]
Weight of [Fe(acac)3] (Theoretical)
1 Al Sharif Y & Al Omari N
Exp.(7) Preparation of [Fe(acac)3] & Ni(acac)2(H2O)2] Chem.323
Percentage yield of [Fe(acac)3]
Draw the structure of complex
Geometry of product
Symmetry point group of product
B.Preparation of Bis(acetylacetonato)diaquanickel (II); [Ni(acac)2(H2O)2]
Write the balanced equation of reaction:
Color of yield
Weight of [Ni(acac)2(H2O)2] ( actual yield (g))
Number of moles of NiSO4.6H2O
Number of moles of acetylacetone.
The Limiting reagent
Number of moles of [Ni(acac)2(H2O)2]
2 Al Sharif Y & Al Omari N
Exp.(7) Preparation of [Fe(acac)3] & Ni(acac)2(H2O)2] Chem.323
Weight of [Ni(acac)2(H2O)2] (Theoretical)
Percentage yield of [Ni(acac)2(H2O)2]
Draw the structure of complex
Geometry of product
Symmetry point group of product
Questions:
Q1. Role of adding the following:
1. Acetylacetone.
2. Sodium hydroxide.
3. Ethanol.
4. Ammonium hydroxide
3 Al Sharif Y & Al Omari N
You might also like
- Example Form 1 Science Paper (With Answers)Document10 pagesExample Form 1 Science Paper (With Answers)Kenny Cheah Soon Lee100% (8)
- Experiment 8 The Preparation of AcetanlideDocument12 pagesExperiment 8 The Preparation of AcetanlideRadhwanNo ratings yet
- Wet Scrubber BulletinDocument8 pagesWet Scrubber BulletinHESuarez100% (1)
- Preparation of BakeliteDocument8 pagesPreparation of Bakeliteusman_uet0868% (19)
- Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International General Certificate of Secondary EducationYasser ZubaidiNo ratings yet
- Chemistry 5070 End of YearDocument7 pagesChemistry 5070 End of Yearsamuelbandamiracle20No ratings yet
- Singapore-Cambridge GCE A Levels Exam Practice Paper IIDocument5 pagesSingapore-Cambridge GCE A Levels Exam Practice Paper IIChong56No ratings yet
- Paper 2 Final (Qs Only)Document17 pagesPaper 2 Final (Qs Only)chuasioklengNo ratings yet
- Bengkel Ambang SPM 2009 Kertas 2Document31 pagesBengkel Ambang SPM 2009 Kertas 2azharsarahNo ratings yet
- Chemistry Form 4 Paper 2Document12 pagesChemistry Form 4 Paper 2nuhrramah2912No ratings yet
- Blio 2Document6 pagesBlio 2karenNo ratings yet
- FY 425 (Chemistry)Document12 pagesFY 425 (Chemistry)Shahzad TNo ratings yet
- CHEMISTRY-P2-Teacher Co KeDocument8 pagesCHEMISTRY-P2-Teacher Co KeASSIGNMENTS PRIORITYNo ratings yet
- IGCSE Chemistry Stoichiometry Worksheet-Empirical FormulaDocument5 pagesIGCSE Chemistry Stoichiometry Worksheet-Empirical Formulaabd rahmanNo ratings yet
- CHAPTER 9 Electrochemistry Structure and Essay 13-19Document5 pagesCHAPTER 9 Electrochemistry Structure and Essay 13-19peter edwardNo ratings yet
- Chem 2Document12 pagesChem 2githukucharles.gcNo ratings yet
- Chemistry Pre-Board STD Sem 2 X Gokuldham High School 2021-22Document8 pagesChemistry Pre-Board STD Sem 2 X Gokuldham High School 2021-22Lmao XdNo ratings yet
- HKALE Mock Paper II (2010)Document14 pagesHKALE Mock Paper II (2010)tiffany5No ratings yet
- 962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)Document12 pages962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)sherry_christyNo ratings yet
- Questions & Answers: For For For For For NEET (UG) - 2020Document21 pagesQuestions & Answers: For For For For For NEET (UG) - 2020Ritu JoharNo ratings yet
- 8-Io Chemistry Paper IIDocument3 pages8-Io Chemistry Paper IIleo3ariNo ratings yet
- Section B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)Document8 pagesSection B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)harrynghomanNo ratings yet
- STPM Trial Negeri Sembilan 2007 Chemistry Paper 2Document21 pagesSTPM Trial Negeri Sembilan 2007 Chemistry Paper 2stuart5051No ratings yet
- Chemistry Paper 2 Sabah STPM 2008 Excel Set 2 (Edu - Joshuatly.com)Document13 pagesChemistry Paper 2 Sabah STPM 2008 Excel Set 2 (Edu - Joshuatly.com)HaRry ChgNo ratings yet
- Chemistry Paper 2 Exam PremiDocument12 pagesChemistry Paper 2 Exam PremiSakinah Saad100% (3)
- Chemistry Paper 1: All Questions Are CompulsoryDocument8 pagesChemistry Paper 1: All Questions Are CompulsoryBhupesh Gupta100% (1)
- Energy Changes Revision ModuleDocument7 pagesEnergy Changes Revision Modulegabrielsuva6No ratings yet
- Chemistry Question PaperDocument4 pagesChemistry Question Paperdrsayan09No ratings yet
- MSS 1718MockPaper2Document8 pagesMSS 1718MockPaper2Kelvin ChowNo ratings yet
- Bengkel Ambang SPM 2009paper 2Document31 pagesBengkel Ambang SPM 2009paper 2Mimi MaliniNo ratings yet
- C Ch-18 Co-Ordination CompoundsDocument7 pagesC Ch-18 Co-Ordination Compoundsmysoftinfo.incNo ratings yet
- F3 Scie ExamDocument6 pagesF3 Scie Examhmatara8No ratings yet
- EstherDocument26 pagesEstherEnock SemweziNo ratings yet
- STPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)Document13 pagesSTPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)kokpin100100% (1)
- CHEMISTRY 2 - Questions N AnswersDocument32 pagesCHEMISTRY 2 - Questions N Answersjuliuskamande844No ratings yet
- Chemistry Form 1 Assignment April 2021 Teacher - Co - .KeDocument5 pagesChemistry Form 1 Assignment April 2021 Teacher - Co - .Kejkipyegon175No ratings yet
- Kibugo - Set ThreeDocument6 pagesKibugo - Set ThreeTalemwa ALFRED KAKORAKINo ratings yet
- HKDSE Chemistry: (Paper 2) Mock Examination 4Document6 pagesHKDSE Chemistry: (Paper 2) Mock Examination 4Vinaigrette HeNo ratings yet
- Chem Form 2 End Term 3Document8 pagesChem Form 2 End Term 3DenisNo ratings yet
- ChemDocument19 pagesChemrussell_mahmoodNo ratings yet
- ch212 Ex5Document6 pagesch212 Ex5nkwaneleNo ratings yet
- Chem pp2Document9 pagesChem pp2ewawireNo ratings yet
- Chemistry Paper 4 October 2004Document11 pagesChemistry Paper 4 October 2004Dean DambazaNo ratings yet
- Chemistry Semester 2 SpecimensDocument6 pagesChemistry Semester 2 SpecimensPB electronicsNo ratings yet
- Chemistry Paper 2 Exam PremiDocument12 pagesChemistry Paper 2 Exam PremiEmily VinciNo ratings yet
- A Level Chemistry Paper 2 Exam 8Document5 pagesA Level Chemistry Paper 2 Exam 8Anthony AndyNo ratings yet
- 2000-2019 Nesa Chemistry Advanced Level-1Document269 pages2000-2019 Nesa Chemistry Advanced Level-1Jeff AlbaNo ratings yet
- Chrmistry Form 4 Chapter 3 Chemical Formulae and EquationsDocument8 pagesChrmistry Form 4 Chapter 3 Chemical Formulae and EquationsEric Wong0% (1)
- Time: 3.00 Hours) : This Question Paper Contains 8 Printed PagesDocument8 pagesTime: 3.00 Hours) : This Question Paper Contains 8 Printed PagesrafikdmeNo ratings yet
- Answer Module 11A-Manufactured Substances in IndustryDocument7 pagesAnswer Module 11A-Manufactured Substances in IndustryYen ZyNo ratings yet
- Chempage - 2022 Chemistry Mock Exam 2022 - Chem - Mock - 2 - QBDocument10 pagesChempage - 2022 Chemistry Mock Exam 2022 - Chem - Mock - 2 - QBChun Kit LauNo ratings yet
- Paper 3 SPM 2011 Mastery PracticesDocument30 pagesPaper 3 SPM 2011 Mastery PracticesaganbasmNo ratings yet
- SK025 KMJ Pre PSPM Set 4 (Question)Document4 pagesSK025 KMJ Pre PSPM Set 4 (Question)2022674978No ratings yet
- f4 CHM Pp2 Et1 Qns Teacher Co KeDocument10 pagesf4 CHM Pp2 Et1 Qns Teacher Co KeRedemptaNo ratings yet
- Chem F4 Mid ExamDocument10 pagesChem F4 Mid ExamYong SiewkuanNo ratings yet
- BECO UACE Chem2Document6 pagesBECO UACE Chem2EMMANUEL BIRUNGINo ratings yet
- Semester 2 Examination CHEMISTRY - Mock Paper (Science Paper 2)Document8 pagesSemester 2 Examination CHEMISTRY - Mock Paper (Science Paper 2)Harshith GowdaNo ratings yet
- Specimen Paper 4Document26 pagesSpecimen Paper 4Thanusha DhanarajNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Experiment No. 2Document6 pagesExperiment No. 2mayankroy9431No ratings yet
- The Mole and Stoichiometry: Multiple Choice QuestionsDocument59 pagesThe Mole and Stoichiometry: Multiple Choice QuestionsRIKI MUHAMMADNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 6Document11 pagesRTS Chemistry SPM Question Bank Chapter 6Vincent Vetter100% (1)
- Carbon and Its CompoundsDocument8 pagesCarbon and Its Compoundsbhumika motiyaniNo ratings yet
- HSC 2016 March ChemistryDocument3 pagesHSC 2016 March ChemistryYSDNo ratings yet
- STPM 2013 Sem 2Document5 pagesSTPM 2013 Sem 2m-4306022No ratings yet
- SBS006 Antibacterial Hand CleanerDocument1 pageSBS006 Antibacterial Hand CleanerFloraNo ratings yet
- 2021 H2 Chemistry Prelim Paper 2Document24 pages2021 H2 Chemistry Prelim Paper 2clarissa yeoNo ratings yet
- Ken338 TDocument13 pagesKen338 TAri DanteNo ratings yet
- CHM 212 Lecture NotesDocument14 pagesCHM 212 Lecture Notesdaniel mwantiNo ratings yet
- 11 Chemistry Solved 01 NewDocument4 pages11 Chemistry Solved 01 NewasdfghjklNo ratings yet
- Cold Process Baking Soda Soap Recipe: MaterialsDocument2 pagesCold Process Baking Soda Soap Recipe: MaterialsMaria José PradoNo ratings yet
- Chemistry Calculations: Type of Calculation Revised?Document100 pagesChemistry Calculations: Type of Calculation Revised?Foxy world 152No ratings yet
- Modified-Qualitative Analysis-QuestionDocument5 pagesModified-Qualitative Analysis-QuestionHimanshu GusainNo ratings yet
- Asakawa 1978Document2 pagesAsakawa 1978Omer MukhtarNo ratings yet
- DENSIDADES DE MASAS DE PARTICULAS. Chap21. PERRYÂ SDocument1 pageDENSIDADES DE MASAS DE PARTICULAS. Chap21. PERRYÂ SStefany Mariela Pineda AyalaNo ratings yet
- Lipids Part 3Document9 pagesLipids Part 3Anonymous 596wAK78eCNo ratings yet
- EDEXCEL A2 CHEMISTRY UNIT 5 January 2011Document24 pagesEDEXCEL A2 CHEMISTRY UNIT 5 January 2011Ghaleb W. MihyarNo ratings yet
- Chemicals Specification ManualDocument139 pagesChemicals Specification Manualvelu.gNo ratings yet
- Redox ReactionDocument3 pagesRedox ReactionPranjal GuptaNo ratings yet
- Ring IndexDocument194 pagesRing IndexlalitarunNo ratings yet
- Chemistry-Orgo II Exam 1 Version A (UD) Answer KeyDocument8 pagesChemistry-Orgo II Exam 1 Version A (UD) Answer KeyNesrine LaradjiNo ratings yet
- Experiment No. 5 Preparation of Aspirin (Initial)Document2 pagesExperiment No. 5 Preparation of Aspirin (Initial)Christine MarcellanaNo ratings yet
- Example of Laboratory ReportDocument5 pagesExample of Laboratory Reportpowasloopas258No ratings yet
- Bleaching 1643433388703Document7 pagesBleaching 1643433388703Ishaan GuptaNo ratings yet
- Macromolecules (Polymers, Carbohydrates, Proteins, and Fats)Document27 pagesMacromolecules (Polymers, Carbohydrates, Proteins, and Fats)Melva SibaraniNo ratings yet
- Mixed Aldol CondensationDocument3 pagesMixed Aldol CondensationSangeeta BansalNo ratings yet