Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
174 viewsVon Richter Reaction
Von Richter Reaction
Uploaded by
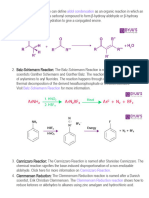
sajal aggarwalThe von Richter reaction involves the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol. This results in a cine substitution reaction, replacing the nitro group with a carboxyl group in an adjacent position on the aromatic ring. For example, p-bromonitrobenzene is converted to m-bromobenzoic acid. The reaction involves nucleophilic aromatic substitution but yields are generally poor to moderate, ranging from 1% to 50%, making it not very synthetically useful. It is named after Victor von Richter who discovered the reaction in 1871.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Leuckart ReactionDocument4 pagesLeuckart ReactionangelofgloryNo ratings yet
- Cataltic Applications of Metal CarbonylsDocument8 pagesCataltic Applications of Metal Carbonylssaud100% (4)
- Stereoselective and Stereospecific ReactionsDocument20 pagesStereoselective and Stereospecific ReactionsHunzalaNo ratings yet
- Corey HouseDocument14 pagesCorey Housepjblk100% (1)
- Fries RearrangementDocument3 pagesFries RearrangementAshuNo ratings yet
- 148 16SCCCH8 2020062412102615Document25 pages148 16SCCCH8 2020062412102615Jawad MughalNo ratings yet
- Benzilic Acid RearrangementDocument4 pagesBenzilic Acid Rearrangementachyuta.1433No ratings yet
- An Are Rob I C DegradationDocument18 pagesAn Are Rob I C DegradationMartuchis EstradaNo ratings yet
- Organic Aldehyde - Isothiocyanate ChemistryDocument244 pagesOrganic Aldehyde - Isothiocyanate Chemistrycarlosazucena1100% (2)
- RearrangementsDocument22 pagesRearrangementsVipul Newaskar100% (1)
- Org. Chem - Rearrangement and Elimination Rxns.Document35 pagesOrg. Chem - Rearrangement and Elimination Rxns.jeevananthampchpa23008No ratings yet
- Coupling Reaction - Wikipedia, The Free EncyclopediaDocument5 pagesCoupling Reaction - Wikipedia, The Free EncyclopediakavitakunduNo ratings yet
- CHE 322 - HydroformylationDocument10 pagesCHE 322 - HydroformylationonkgopotsemahiloNo ratings yet
- The Beckmann RearrangementDocument12 pagesThe Beckmann RearrangementSukumar PaniNo ratings yet
- Reaksi Kopling Merupakan Reaksi Penggabungan Rantai KarbonDocument9 pagesReaksi Kopling Merupakan Reaksi Penggabungan Rantai KarbonfikarisvitaNo ratings yet
- Pinacol RearrangementDocument2 pagesPinacol RearrangementkarinadegomaNo ratings yet
- Reaction Mechanism NewDocument25 pagesReaction Mechanism NewPUNEET KANNAUJIYANo ratings yet
- Upct HW Notes (Gtustudy - In)Document242 pagesUpct HW Notes (Gtustudy - In)Yash PatelNo ratings yet
- Unit Process in Chemical Technology (UPCT)Document14 pagesUnit Process in Chemical Technology (UPCT)devendravankar883No ratings yet
- 10 Carbonium Ion RearrangementsDocument56 pages10 Carbonium Ion Rearrangementssafiabarkat5No ratings yet
- Junaid and ImranDocument29 pagesJunaid and ImranJunaid KhanNo ratings yet
- Wittig Reaction SibiDocument4 pagesWittig Reaction SibivinaybharadwajbsNo ratings yet
- Name Reactions1Document4 pagesName Reactions1Vijaya RajuNo ratings yet
- Dehydrogenation by Heterogeneous CatalystsDocument52 pagesDehydrogenation by Heterogeneous CatalystsNur GeehanNo ratings yet
- 1860 5397 12 162 PDFDocument102 pages1860 5397 12 162 PDFIta AzmizakiyahNo ratings yet
- Chemical Synthesis Is A PurposefulDocument10 pagesChemical Synthesis Is A PurposefulpraveenNo ratings yet
- Assignment 624Document10 pagesAssignment 6241A12MUHAMMAD HAFIY IMRANNo ratings yet
- Wurtz-Fittig Reaction 0Document6 pagesWurtz-Fittig Reaction 0Ritera PeiriaNo ratings yet
- ArticuloDocument149 pagesArticuloRaydi FuenmayorNo ratings yet
- Week 3 Reactions of AlkenesDocument39 pagesWeek 3 Reactions of AlkenesKate Arianne CabreraNo ratings yet
- ReactionDocument54 pagesReactionAhmed ImranNo ratings yet
- 8.3 THE S 2 Mechanism OF Nucleophilic Substitution: 306 Chapter EightDocument1 page8.3 THE S 2 Mechanism OF Nucleophilic Substitution: 306 Chapter EightFrista IrwanindaNo ratings yet
- Chemical Reaction - WikipediaDocument20 pagesChemical Reaction - WikipediakamaalNo ratings yet
- Introduction To Organic Reaction For Pharmacy StudentsDocument90 pagesIntroduction To Organic Reaction For Pharmacy Studentszekarias wondafrashNo ratings yet
- Petroleum Microbiology Group 5Document7 pagesPetroleum Microbiology Group 5david ojNo ratings yet
- Chemical ReactionsDocument5 pagesChemical ReactionszzaanNo ratings yet
- Outline An Experiment You Performed An Alkanol and Evaluate The Appropriateness Validity of Your ExperimentDocument4 pagesOutline An Experiment You Performed An Alkanol and Evaluate The Appropriateness Validity of Your ExperimentRickyNo ratings yet
- Environmental Chemistry: Lectures 4 To 5Document50 pagesEnvironmental Chemistry: Lectures 4 To 5BHENCHO1001No ratings yet
- Robinson Annulation 1Document6 pagesRobinson Annulation 1Auwal ShehuNo ratings yet
- Applied Catalysis B: EnvironmentalDocument8 pagesApplied Catalysis B: EnvironmentalJocsan AxelNo ratings yet
- Nef ReactionDocument3 pagesNef ReactionSubhabrata MabhaiNo ratings yet
- Nitration - WikipediaDocument7 pagesNitration - WikipediaAhmed ranaNo ratings yet
- The Most Well-Known Rearrangements in Organic Chemistry at HandDocument32 pagesThe Most Well-Known Rearrangements in Organic Chemistry at HandAnkit JagetiaNo ratings yet
- Enamines and YlidesDocument18 pagesEnamines and YlidesVijay Pradhan100% (1)
- HydroborationDocument5 pagesHydroborationTwasalifya SiwaleNo ratings yet
- 12-Aliphatic Nucleophilic Substitution PDFDocument68 pages12-Aliphatic Nucleophilic Substitution PDFVenkatraj Gowdas100% (1)
- The Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoDocument43 pagesThe Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoLuis J. RomeroNo ratings yet
- Organic Reactions v1Document396 pagesOrganic Reactions v1rhozab100% (5)
- Oxidation - Oxidation Reactions Are Some of The Most Common Xenobiotic Transformations Occurring inDocument3 pagesOxidation - Oxidation Reactions Are Some of The Most Common Xenobiotic Transformations Occurring inKevin SangNo ratings yet
- Reactions of Synthetic ImportanceDocument28 pagesReactions of Synthetic ImportanceRx Nadeem ChhipaNo ratings yet
- Aromatic HydrocarbonsDocument37 pagesAromatic HydrocarbonsMae Rose PicaranaNo ratings yet
- The Chemical Recylinf of PUDocument21 pagesThe Chemical Recylinf of PU14021292No ratings yet
- Acid CatalysisDocument16 pagesAcid CatalysisTayyaba SadaqNo ratings yet
- Presentation by Koushik AlifDocument17 pagesPresentation by Koushik Alifalifkoushik4No ratings yet
- Domino and Intramolecular Rearrangement Reactions as Advanced Synthetic Methods in GlycoscienceFrom EverandDomino and Intramolecular Rearrangement Reactions as Advanced Synthetic Methods in GlycoscienceNo ratings yet
- Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural ProductsFrom EverandSynthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural ProductsAlbert PadwaNo ratings yet
- The Development of Catalysis: A History of Key Processes and Personas in Catalytic Science and TechnologyFrom EverandThe Development of Catalysis: A History of Key Processes and Personas in Catalytic Science and TechnologyNo ratings yet
- Organic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981From EverandOrganic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981A. C. KnipeNo ratings yet
- Chapter21 - Vector - 3D PDFDocument72 pagesChapter21 - Vector - 3D PDFsajal aggarwalNo ratings yet
- Chapter01 - Logarithm PDFDocument22 pagesChapter01 - Logarithm PDFsajal aggarwalNo ratings yet
- Biology: Study Package Zenith / Octagon Class XDocument19 pagesBiology: Study Package Zenith / Octagon Class Xsajal aggarwalNo ratings yet
- 05 - Why Do We Fall IllDocument13 pages05 - Why Do We Fall Illsajal aggarwalNo ratings yet
- Ntse Material For PreparationDocument5 pagesNtse Material For Preparationsajal aggarwalNo ratings yet
- Was A Period When The Deccan and The South Became PowerfulDocument1 pageWas A Period When The Deccan and The South Became Powerfulsajal aggarwalNo ratings yet
- Under Bimbisara and Ajat Shatru and Shisunanga and Nanda DynastyDocument1 pageUnder Bimbisara and Ajat Shatru and Shisunanga and Nanda Dynastysajal aggarwalNo ratings yet
- Early Historic Period: The Stone AgeDocument1 pageEarly Historic Period: The Stone Agesajal aggarwalNo ratings yet
- Ancient India Timeli: MahajanapadasDocument1 pageAncient India Timeli: Mahajanapadassajal aggarwalNo ratings yet
- 04 - Improvement in Food ResourcesDocument18 pages04 - Improvement in Food Resourcessajal aggarwalNo ratings yet
- 05 - Our EnvironmentDocument12 pages05 - Our Environmentsajal aggarwalNo ratings yet
- GD Goenka Public School Class Ix Revision Worksheet Chapter-8 & 9 DATE-28.01.2020Document2 pagesGD Goenka Public School Class Ix Revision Worksheet Chapter-8 & 9 DATE-28.01.2020sajal aggarwalNo ratings yet
- G.D.Goenka Public School Practice Sheet - 2 Quadrilaterals Class IxDocument2 pagesG.D.Goenka Public School Practice Sheet - 2 Quadrilaterals Class Ixsajal aggarwalNo ratings yet
- GD Goenka Public School Class Ix Revision Worksheet CHAPTER-6 &7Document2 pagesGD Goenka Public School Class Ix Revision Worksheet CHAPTER-6 &7sajal aggarwalNo ratings yet
- G D Goenka Public School, Sector-22 Rohini Class-Ix Subject-Geography Chapter-6-PopulationDocument4 pagesG D Goenka Public School, Sector-22 Rohini Class-Ix Subject-Geography Chapter-6-Populationsajal aggarwalNo ratings yet
- Maths 3Document1 pageMaths 3sajal aggarwalNo ratings yet
- G.D.Goenka Public School Practice Sheet - 1 Quadrilaterals Class IxDocument3 pagesG.D.Goenka Public School Practice Sheet - 1 Quadrilaterals Class Ixsajal aggarwalNo ratings yet
- Geomorphic Characteristics: Plateau, Extensive Area ofDocument1 pageGeomorphic Characteristics: Plateau, Extensive Area ofsajal aggarwalNo ratings yet
- G.D.Goenka Public School, Sec-22, Rohini Chapter-4: Structure of Atom Worksheet-2Document3 pagesG.D.Goenka Public School, Sec-22, Rohini Chapter-4: Structure of Atom Worksheet-2sajal aggarwalNo ratings yet
- G. D. Goenka Public School IX English Packing - Jerome K. JeromeDocument1 pageG. D. Goenka Public School IX English Packing - Jerome K. Jeromesajal aggarwalNo ratings yet
- English 1Document1 pageEnglish 1sajal aggarwalNo ratings yet
- Hills Geology Depression U-Shaped Geography: Valley or River Valley, Is Usually V-Shaped. The Exact Shape WillDocument1 pageHills Geology Depression U-Shaped Geography: Valley or River Valley, Is Usually V-Shaped. The Exact Shape Willsajal aggarwalNo ratings yet
- Relief PlateausDocument2 pagesRelief Plateaussajal aggarwalNo ratings yet
Von Richter Reaction
Von Richter Reaction
Uploaded by
sajal aggarwal0 ratings0% found this document useful (0 votes)
174 views1 pageThe von Richter reaction involves the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol. This results in a cine substitution reaction, replacing the nitro group with a carboxyl group in an adjacent position on the aromatic ring. For example, p-bromonitrobenzene is converted to m-bromobenzoic acid. The reaction involves nucleophilic aromatic substitution but yields are generally poor to moderate, ranging from 1% to 50%, making it not very synthetically useful. It is named after Victor von Richter who discovered the reaction in 1871.
Original Description:
CHEMISTRY
Original Title
Von Richter reaction
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe von Richter reaction involves the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol. This results in a cine substitution reaction, replacing the nitro group with a carboxyl group in an adjacent position on the aromatic ring. For example, p-bromonitrobenzene is converted to m-bromobenzoic acid. The reaction involves nucleophilic aromatic substitution but yields are generally poor to moderate, ranging from 1% to 50%, making it not very synthetically useful. It is named after Victor von Richter who discovered the reaction in 1871.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
174 views1 pageVon Richter Reaction
Von Richter Reaction
Uploaded by
sajal aggarwalThe von Richter reaction involves the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol. This results in a cine substitution reaction, replacing the nitro group with a carboxyl group in an adjacent position on the aromatic ring. For example, p-bromonitrobenzene is converted to m-bromobenzoic acid. The reaction involves nucleophilic aromatic substitution but yields are generally poor to moderate, ranging from 1% to 50%, making it not very synthetically useful. It is named after Victor von Richter who discovered the reaction in 1871.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
Von Richter reaction
From Wikipedia, the free encyclopedia
Jump to navigation Jump to search
The von Richter reaction, also named von Richter rearrangement, is a name reaction in the
organic chemistry. It is named after Victor von Richter, who discovered this reaction in year 1871. It
is the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol to give the
product of cine substitution (ring substitution resulting in the entering group positioned adjacent to
the previous location of the leaving group) by a carboxyl group.[1][2][3] Although it is not generally
synthetically useful due to the low chemical yield and formation of numerous side products, its
mechanism was of considerable interest, eluding chemists for almost 100 years before the currently
accepted one was proposed.
General Reaction Scheme[edit]
The reaction below shows the classic example of the conversion of p-bromonitrobenzene into m-
bromobenzoic acid.[4]
The reaction is a type of nucleophilic aromatic substitution.[4] Besides the bromo derivative, chlorine-
and iodine-substituted nitroarenes, as well as more highly substituted derivatives, could also be used
as substrates of this reaction. However, yields are generally poor to moderate, with reported
percentage yields ranging from 1% to 50%.[5][6]
You might also like
- Leuckart ReactionDocument4 pagesLeuckart ReactionangelofgloryNo ratings yet
- Cataltic Applications of Metal CarbonylsDocument8 pagesCataltic Applications of Metal Carbonylssaud100% (4)
- Stereoselective and Stereospecific ReactionsDocument20 pagesStereoselective and Stereospecific ReactionsHunzalaNo ratings yet
- Corey HouseDocument14 pagesCorey Housepjblk100% (1)
- Fries RearrangementDocument3 pagesFries RearrangementAshuNo ratings yet
- 148 16SCCCH8 2020062412102615Document25 pages148 16SCCCH8 2020062412102615Jawad MughalNo ratings yet
- Benzilic Acid RearrangementDocument4 pagesBenzilic Acid Rearrangementachyuta.1433No ratings yet
- An Are Rob I C DegradationDocument18 pagesAn Are Rob I C DegradationMartuchis EstradaNo ratings yet
- Organic Aldehyde - Isothiocyanate ChemistryDocument244 pagesOrganic Aldehyde - Isothiocyanate Chemistrycarlosazucena1100% (2)
- RearrangementsDocument22 pagesRearrangementsVipul Newaskar100% (1)
- Org. Chem - Rearrangement and Elimination Rxns.Document35 pagesOrg. Chem - Rearrangement and Elimination Rxns.jeevananthampchpa23008No ratings yet
- Coupling Reaction - Wikipedia, The Free EncyclopediaDocument5 pagesCoupling Reaction - Wikipedia, The Free EncyclopediakavitakunduNo ratings yet
- CHE 322 - HydroformylationDocument10 pagesCHE 322 - HydroformylationonkgopotsemahiloNo ratings yet
- The Beckmann RearrangementDocument12 pagesThe Beckmann RearrangementSukumar PaniNo ratings yet
- Reaksi Kopling Merupakan Reaksi Penggabungan Rantai KarbonDocument9 pagesReaksi Kopling Merupakan Reaksi Penggabungan Rantai KarbonfikarisvitaNo ratings yet
- Pinacol RearrangementDocument2 pagesPinacol RearrangementkarinadegomaNo ratings yet
- Reaction Mechanism NewDocument25 pagesReaction Mechanism NewPUNEET KANNAUJIYANo ratings yet
- Upct HW Notes (Gtustudy - In)Document242 pagesUpct HW Notes (Gtustudy - In)Yash PatelNo ratings yet
- Unit Process in Chemical Technology (UPCT)Document14 pagesUnit Process in Chemical Technology (UPCT)devendravankar883No ratings yet
- 10 Carbonium Ion RearrangementsDocument56 pages10 Carbonium Ion Rearrangementssafiabarkat5No ratings yet
- Junaid and ImranDocument29 pagesJunaid and ImranJunaid KhanNo ratings yet
- Wittig Reaction SibiDocument4 pagesWittig Reaction SibivinaybharadwajbsNo ratings yet
- Name Reactions1Document4 pagesName Reactions1Vijaya RajuNo ratings yet
- Dehydrogenation by Heterogeneous CatalystsDocument52 pagesDehydrogenation by Heterogeneous CatalystsNur GeehanNo ratings yet
- 1860 5397 12 162 PDFDocument102 pages1860 5397 12 162 PDFIta AzmizakiyahNo ratings yet
- Chemical Synthesis Is A PurposefulDocument10 pagesChemical Synthesis Is A PurposefulpraveenNo ratings yet
- Assignment 624Document10 pagesAssignment 6241A12MUHAMMAD HAFIY IMRANNo ratings yet
- Wurtz-Fittig Reaction 0Document6 pagesWurtz-Fittig Reaction 0Ritera PeiriaNo ratings yet
- ArticuloDocument149 pagesArticuloRaydi FuenmayorNo ratings yet
- Week 3 Reactions of AlkenesDocument39 pagesWeek 3 Reactions of AlkenesKate Arianne CabreraNo ratings yet
- ReactionDocument54 pagesReactionAhmed ImranNo ratings yet
- 8.3 THE S 2 Mechanism OF Nucleophilic Substitution: 306 Chapter EightDocument1 page8.3 THE S 2 Mechanism OF Nucleophilic Substitution: 306 Chapter EightFrista IrwanindaNo ratings yet
- Chemical Reaction - WikipediaDocument20 pagesChemical Reaction - WikipediakamaalNo ratings yet
- Introduction To Organic Reaction For Pharmacy StudentsDocument90 pagesIntroduction To Organic Reaction For Pharmacy Studentszekarias wondafrashNo ratings yet
- Petroleum Microbiology Group 5Document7 pagesPetroleum Microbiology Group 5david ojNo ratings yet
- Chemical ReactionsDocument5 pagesChemical ReactionszzaanNo ratings yet
- Outline An Experiment You Performed An Alkanol and Evaluate The Appropriateness Validity of Your ExperimentDocument4 pagesOutline An Experiment You Performed An Alkanol and Evaluate The Appropriateness Validity of Your ExperimentRickyNo ratings yet
- Environmental Chemistry: Lectures 4 To 5Document50 pagesEnvironmental Chemistry: Lectures 4 To 5BHENCHO1001No ratings yet
- Robinson Annulation 1Document6 pagesRobinson Annulation 1Auwal ShehuNo ratings yet
- Applied Catalysis B: EnvironmentalDocument8 pagesApplied Catalysis B: EnvironmentalJocsan AxelNo ratings yet
- Nef ReactionDocument3 pagesNef ReactionSubhabrata MabhaiNo ratings yet
- Nitration - WikipediaDocument7 pagesNitration - WikipediaAhmed ranaNo ratings yet
- The Most Well-Known Rearrangements in Organic Chemistry at HandDocument32 pagesThe Most Well-Known Rearrangements in Organic Chemistry at HandAnkit JagetiaNo ratings yet
- Enamines and YlidesDocument18 pagesEnamines and YlidesVijay Pradhan100% (1)
- HydroborationDocument5 pagesHydroborationTwasalifya SiwaleNo ratings yet
- 12-Aliphatic Nucleophilic Substitution PDFDocument68 pages12-Aliphatic Nucleophilic Substitution PDFVenkatraj Gowdas100% (1)
- The Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoDocument43 pagesThe Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoLuis J. RomeroNo ratings yet
- Organic Reactions v1Document396 pagesOrganic Reactions v1rhozab100% (5)
- Oxidation - Oxidation Reactions Are Some of The Most Common Xenobiotic Transformations Occurring inDocument3 pagesOxidation - Oxidation Reactions Are Some of The Most Common Xenobiotic Transformations Occurring inKevin SangNo ratings yet
- Reactions of Synthetic ImportanceDocument28 pagesReactions of Synthetic ImportanceRx Nadeem ChhipaNo ratings yet
- Aromatic HydrocarbonsDocument37 pagesAromatic HydrocarbonsMae Rose PicaranaNo ratings yet
- The Chemical Recylinf of PUDocument21 pagesThe Chemical Recylinf of PU14021292No ratings yet
- Acid CatalysisDocument16 pagesAcid CatalysisTayyaba SadaqNo ratings yet
- Presentation by Koushik AlifDocument17 pagesPresentation by Koushik Alifalifkoushik4No ratings yet
- Domino and Intramolecular Rearrangement Reactions as Advanced Synthetic Methods in GlycoscienceFrom EverandDomino and Intramolecular Rearrangement Reactions as Advanced Synthetic Methods in GlycoscienceNo ratings yet
- Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural ProductsFrom EverandSynthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural ProductsAlbert PadwaNo ratings yet
- The Development of Catalysis: A History of Key Processes and Personas in Catalytic Science and TechnologyFrom EverandThe Development of Catalysis: A History of Key Processes and Personas in Catalytic Science and TechnologyNo ratings yet
- Organic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981From EverandOrganic Reaction Mechanisms 1981: An annual survey covering the literature dated December 1980 through November 1981A. C. KnipeNo ratings yet
- Chapter21 - Vector - 3D PDFDocument72 pagesChapter21 - Vector - 3D PDFsajal aggarwalNo ratings yet
- Chapter01 - Logarithm PDFDocument22 pagesChapter01 - Logarithm PDFsajal aggarwalNo ratings yet
- Biology: Study Package Zenith / Octagon Class XDocument19 pagesBiology: Study Package Zenith / Octagon Class Xsajal aggarwalNo ratings yet
- 05 - Why Do We Fall IllDocument13 pages05 - Why Do We Fall Illsajal aggarwalNo ratings yet
- Ntse Material For PreparationDocument5 pagesNtse Material For Preparationsajal aggarwalNo ratings yet
- Was A Period When The Deccan and The South Became PowerfulDocument1 pageWas A Period When The Deccan and The South Became Powerfulsajal aggarwalNo ratings yet
- Under Bimbisara and Ajat Shatru and Shisunanga and Nanda DynastyDocument1 pageUnder Bimbisara and Ajat Shatru and Shisunanga and Nanda Dynastysajal aggarwalNo ratings yet
- Early Historic Period: The Stone AgeDocument1 pageEarly Historic Period: The Stone Agesajal aggarwalNo ratings yet
- Ancient India Timeli: MahajanapadasDocument1 pageAncient India Timeli: Mahajanapadassajal aggarwalNo ratings yet
- 04 - Improvement in Food ResourcesDocument18 pages04 - Improvement in Food Resourcessajal aggarwalNo ratings yet
- 05 - Our EnvironmentDocument12 pages05 - Our Environmentsajal aggarwalNo ratings yet
- GD Goenka Public School Class Ix Revision Worksheet Chapter-8 & 9 DATE-28.01.2020Document2 pagesGD Goenka Public School Class Ix Revision Worksheet Chapter-8 & 9 DATE-28.01.2020sajal aggarwalNo ratings yet
- G.D.Goenka Public School Practice Sheet - 2 Quadrilaterals Class IxDocument2 pagesG.D.Goenka Public School Practice Sheet - 2 Quadrilaterals Class Ixsajal aggarwalNo ratings yet
- GD Goenka Public School Class Ix Revision Worksheet CHAPTER-6 &7Document2 pagesGD Goenka Public School Class Ix Revision Worksheet CHAPTER-6 &7sajal aggarwalNo ratings yet
- G D Goenka Public School, Sector-22 Rohini Class-Ix Subject-Geography Chapter-6-PopulationDocument4 pagesG D Goenka Public School, Sector-22 Rohini Class-Ix Subject-Geography Chapter-6-Populationsajal aggarwalNo ratings yet
- Maths 3Document1 pageMaths 3sajal aggarwalNo ratings yet
- G.D.Goenka Public School Practice Sheet - 1 Quadrilaterals Class IxDocument3 pagesG.D.Goenka Public School Practice Sheet - 1 Quadrilaterals Class Ixsajal aggarwalNo ratings yet
- Geomorphic Characteristics: Plateau, Extensive Area ofDocument1 pageGeomorphic Characteristics: Plateau, Extensive Area ofsajal aggarwalNo ratings yet
- G.D.Goenka Public School, Sec-22, Rohini Chapter-4: Structure of Atom Worksheet-2Document3 pagesG.D.Goenka Public School, Sec-22, Rohini Chapter-4: Structure of Atom Worksheet-2sajal aggarwalNo ratings yet
- G. D. Goenka Public School IX English Packing - Jerome K. JeromeDocument1 pageG. D. Goenka Public School IX English Packing - Jerome K. Jeromesajal aggarwalNo ratings yet
- English 1Document1 pageEnglish 1sajal aggarwalNo ratings yet
- Hills Geology Depression U-Shaped Geography: Valley or River Valley, Is Usually V-Shaped. The Exact Shape WillDocument1 pageHills Geology Depression U-Shaped Geography: Valley or River Valley, Is Usually V-Shaped. The Exact Shape Willsajal aggarwalNo ratings yet
- Relief PlateausDocument2 pagesRelief Plateaussajal aggarwalNo ratings yet