Professional Documents
Culture Documents
10-Water Quality and Contamination
10-Water Quality and Contamination
Uploaded by
Harith Emaad0 ratings0% found this document useful (0 votes)
20 views18 pages10-Water quality and contamination
Original Title
10-Water quality and contamination
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document10-Water quality and contamination
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
20 views18 pages10-Water Quality and Contamination
10-Water Quality and Contamination
Uploaded by
Harith Emaad10-Water quality and contamination
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 18
Water quality and contamination
The surface and ground water quality is one of the most
important factors in water resources management.
Hydrochemical study is concentrated on studying the
physical and chemical parameters of water. These
characteristics of water determine its usefulness for
commercial, industrial, agricultural, domestic and
drinking water purposes. These characteristics
includes:
1- Physical characters.
Physical characters includes Temperature (T),
Hydrogen number (pH), Total dissolved solids (TDS),
Electrical conductivity (EC) and Turbidity.
Chemical parameters includes analyses of cations (Na+,
K+, Ca2+, Mg2+), anions(Cl-, HCO3-, NO3-, SO42-,, F-),
heavy metals like ( Pb, Zn, Cr, Cd, Cu) and trace
metals like (Rb, Ti, Fe, Mn, …), unstable volatiles
(CO2, H2S, O2) and organic materials.
Physical properties of water:
1- Temperature:
Temperature is one of the conservative properties of
rivers and especially the reservoir water. It affects the
density and viscosity properties of water.
and also effects the geochemical and chemical
reactions.
2- Hydrogen Number (pH): is a numeric scale
used to specify the acidity or basicity of
an aqueous solution.
pH is one of the most important operational
quality parameters of water and wastewater.
The pH of water that were not affected by
contamination typically ranges between 6.5
and 8.0 according to WHO standard units.
)Electrical Conductivity (EC-3
Electrical conductivity (EC) is the ability of 1
cm3 of water to conduct an electric current at a
standard temperature of 25oC that depends on
the total amount of soluble salts.
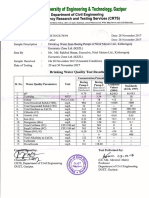
Table below showing the IQS (Iraqi), 2009 and
WHO, 2008 standards for solubility of water
for human drinking.
IQS and WHO standards for Human drinking
4- Total Dissolved Solids (TDS)
Total Dissolved Solids (TDS) or salinity : It
represents the total amount of solids remaining
when a water sample evaporates to dryness.
High TDS values are related to electrical
conductivity (EC) levels, According to Joseph
(2009), the relationship between EC and TDS
is expressed as:
TDS = EC * F; where F is a factor its value is
o.6.
• TDS is reported in units of parts per million
(ppm) or (mg/l). Some dissolved solids
originate from sources such as and industrial
waste and sewage. Other sources are from
runoff, fertilizers used on farms. Any change
in the pH values creates changes in TDS
values. When the pH values decrease due to
bacterial activity, it causes an increase in the
TDS value.
)Classification of water according to TDS in (mg/l
Water Class )Range (ppm
Fresh water 0-1000
Slightly brackish water 1000-3000
brackish 3000-10,000
Salty water 10,000-100,000
Brine water More than 100,000
Turbidity-5
Turbidity is the amount of suspended
particulate matter in water which is caused
by clay, silt, fine organic and inorganic
matter and microorganisms.
Turbidity measures the scattering of light on
the suspended particles in the water using
nephelometric turbidity unit (NTU) and (5
NTU) is usually acceptable for drinking
according to (WHO, 2006).
Chemical Parameters
The chemistry of water is detected mainly by ion
concentrations. Composition of ions is governed by
chemical weathering process and human activity. The
ionic composition of surface and ground water is
governed by exchanges with the underlying material
of the drainage basin and with atmospheric
precipitation. Human activities within the drainage
basin also influence the ionic composition, by altering
discharge regimes and transport of particular matter
across the landscape.
Major Cations
1- Calcium ion (Ca2+) and Magnesium (Mg2+) :
The calcium ion is the dominant cation in water
samples. The main source of Ca2+ is the chemical
weathering of rocks and minerals, such as limestone
and dolomite which are dominant in the area. The
solubility of the carbonate rocks increases in the
presence of solutions rich in dissolved carbon
dioxides. Therefore, the percolated water that are
enriched with CO2 from the atmosphere.
3- Sodium (Na+):
The Source of this ion is the Na bearing rock-
forming minerals such as Halite and
plagioclase. Human activities also can have a
significant influence on the concentration of
sodium in surface water and groundwater.
Sodium concentration is important in
classifying irrigation water, because it reacts
with soil to reduce its permeability.
Potassium ion (K+):
Generally speaking, the low concentration of K +
in water is related to the stability of potassium-
bearing alumina-silicate minerals. These low
concentrations of K attributed to the limited
occurrences of K-bearing rock-forming
minerals are silicate rocks like the feldspars
orthoclase and microcline, micas, and the
feldspathoid leucite. In most natural water, the
concentration of potassium is much lower than
the concentration of sodium.
)Total Hardness (T.H
Total Hardness denotes the concentration of
calcium and magnesium in water, which are
able to precipitate when it is heated. hardness
for water samples are determined by using the
following equation:
Total Hardness (T.H) = 2.497 (Ca mg/l) + 4.115 (Mg mg/l)
Total Hardness Classification
)T.H (mg/l CaCO3 Type
0-70 Very soft water
7-140 Soft water
200 -14 Moderately hard
water
20-300 Fairly hard water
30-500 Hard water
500 < Very hard water
Major Anions
)-1. Bicarbonate (HCO3
Bicarbonate and carbonate are the producer of
alkalinity which is the capacity for solutes and
its contains to react with acids. The principal
source of carbon dioxide species that produce
alkalinity in surface or ground water is the
CO2 gas fraction of the atmosphere.
2. Sulfate (SO42-)
The natural sources are from (dissolution of
evaporated rocks like Gypsum and
Anhydrate), or may be derived from chemical
fertilizers, The presence of sulfate in drinking-
water can cause noticeable taste. The sources
of sulfate are from the secondary gypsum
which precipitates in the caverns and fissures
of geological formations.
3- Chloride
Natural sources of salt in water resources include
geologic deposits containing halite, or saline
groundwater, It is also abundant in minerals of
igneous rocks like apatite and feldspathoid.
Heavy Metals
Heavy elements are mainly derived from the
weathering of rocks and human activities. They are a
special group of trace elements which have been
shown to create definite health hazards when taken up
by plants. This group is included, Fe, Mn, As, Zn,
Cr, Cd, Ni, Cu, Pb and Co
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5822)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Erbil Technology Institute Road Construction DeptDocument12 pagesErbil Technology Institute Road Construction DeptHarith EmaadNo ratings yet
- Qorivva MPC56xx Flash Programming Through Nexus/JTAG: Application NoteDocument44 pagesQorivva MPC56xx Flash Programming Through Nexus/JTAG: Application NoteHarith EmaadNo ratings yet
- Khanda Habeeb RaheemDocument12 pagesKhanda Habeeb RaheemHarith EmaadNo ratings yet
- Firas Fawzi Jirjees: Assistant Lecturer at Erbil Polytechnic University Consultant Civil Engineer (M.Sc. Degree)Document3 pagesFiras Fawzi Jirjees: Assistant Lecturer at Erbil Polytechnic University Consultant Civil Engineer (M.Sc. Degree)Harith EmaadNo ratings yet
- CH7. Eng. Hydr. Lecture Notes FainalDocument56 pagesCH7. Eng. Hydr. Lecture Notes FainalHarith EmaadNo ratings yet
- L S F D 1994 N eDocument15 pagesL S F D 1994 N eHarith EmaadNo ratings yet
- Datasets For Liquefaction Case Studies of Gravelly Soils During The 2008 Wenchuan EarthquakeDocument11 pagesDatasets For Liquefaction Case Studies of Gravelly Soils During The 2008 Wenchuan EarthquakeHarith EmaadNo ratings yet
- Precipitation Lec.3Document13 pagesPrecipitation Lec.3Harith EmaadNo ratings yet
- Evaporation and Evapotranspiration 4Document17 pagesEvaporation and Evapotranspiration 4Harith EmaadNo ratings yet
- 4-Unit Hydrograph, FloodDocument12 pages4-Unit Hydrograph, FloodHarith Emaad100% (1)
- RGDRGFDDocument197 pagesRGDRGFDHarith EmaadNo ratings yet
- Hot Mix Asphalt-Production ProcessDocument16 pagesHot Mix Asphalt-Production ProcessHarith EmaadNo ratings yet
- BU UkahDocument13 pagesBU UkahElizabeth FigueroaNo ratings yet
- Electrol Condct TheryDocument0 pagesElectrol Condct TheryBogdan BulgariuNo ratings yet
- Heating Element Without Thermostatic Head With Fixed Wiring, Nickel-Plated, With G 6/4" ThreadDocument2 pagesHeating Element Without Thermostatic Head With Fixed Wiring, Nickel-Plated, With G 6/4" ThreadmacakafkaNo ratings yet
- Boiler Water ConditioningDocument3 pagesBoiler Water ConditioningShahin AfrozNo ratings yet
- L11-Properties of Oilfield WatersDocument30 pagesL11-Properties of Oilfield WatersLawrenceLopezNo ratings yet
- Manual de Usuario Peachimetro Orion 3 Star PH Portable PDFDocument299 pagesManual de Usuario Peachimetro Orion 3 Star PH Portable PDFELVIS ALFREDO ALVARADO PARAHUATYNo ratings yet
- 25 SWRO MF DatasheetDocument4 pages25 SWRO MF DatasheetAlb FirNo ratings yet
- Water QualityDocument34 pagesWater QualitySarim ChNo ratings yet
- Final Report 5Document5 pagesFinal Report 5Alyssa OrtegaNo ratings yet
- Practica Lab Induced Gas FlotationDocument19 pagesPractica Lab Induced Gas Flotationneurolepsia3790No ratings yet
- Lake EcosystemDocument31 pagesLake EcosystemEllaine Larren RazonNo ratings yet
- SMEDA Mineral Water (Water Bottling Plant)Document29 pagesSMEDA Mineral Water (Water Bottling Plant)Xain AliNo ratings yet
- TSSBackground PDFDocument2 pagesTSSBackground PDFemiNo ratings yet
- Raw Water Analysis ReportDocument1 pageRaw Water Analysis ReportSajib Chandra RoyNo ratings yet
- Gravimetric Analysis 2021Document25 pagesGravimetric Analysis 2021Kakar KakarNo ratings yet
- Building Resilience On Water Quality Management Through Grey - 2018 - Procedia EDocument8 pagesBuilding Resilience On Water Quality Management Through Grey - 2018 - Procedia ENicole Louise RiveraNo ratings yet
- Fowzia Adiyahba PDFDocument86 pagesFowzia Adiyahba PDFGlomarie GonayonNo ratings yet
- Floccin Brochure 2012Document6 pagesFloccin Brochure 2012Jorge MontanoNo ratings yet
- Orion Star Thermo Scientific4 PDFDocument72 pagesOrion Star Thermo Scientific4 PDFOmar Campuzano CalderónNo ratings yet
- Working Principle of Electronic Water DescalerDocument13 pagesWorking Principle of Electronic Water DescalerJack LamNo ratings yet
- Journal of Global Biosciences: Research PaperDocument12 pagesJournal of Global Biosciences: Research PaperAndrew PubgNo ratings yet
- DAO 35 Series 1990 Revised Effluent Regulations of 1990Document12 pagesDAO 35 Series 1990 Revised Effluent Regulations of 1990Ronald O.No ratings yet
- 02 - Create - ROC-3313Document68 pages02 - Create - ROC-3313Heinium DannemannNo ratings yet
- Report On Kusum Sarovar FinalDocument7 pagesReport On Kusum Sarovar FinalVinita TiwaryNo ratings yet
- Kallai River Water Pollution 0 TH Review (2) TDocument43 pagesKallai River Water Pollution 0 TH Review (2) Tpratheesh thekedanNo ratings yet
- DM PlantDocument13 pagesDM PlantAjay Kasture100% (3)
- Water QualityDocument4 pagesWater QualityRamsathayaNo ratings yet
- Poster 22216 PDFDocument1 pagePoster 22216 PDFHaneen AbdelrahmanNo ratings yet
- Purification of Grey Water Using The Natural MethodDocument7 pagesPurification of Grey Water Using The Natural MethodMamta AgarwalNo ratings yet
- Excerpts From Lecture Notes of Professor M. Ashraf Ali, BUETDocument36 pagesExcerpts From Lecture Notes of Professor M. Ashraf Ali, BUETmanowarNo ratings yet