Professional Documents
Culture Documents
Chalk Chromatography
Chalk Chromatography
Uploaded by
Rojo John0 ratings0% found this document useful (0 votes)
248 views1 pageChromatography is a technique used to separate mixtures into individual components. In 1906, Mikhail Tswett used calcium carbonate (chalk) to separate plant pigments by passing them through a chalk-filled glass tube with petroleum ether, resulting in distinct colored zones. This technique, known as column chromatography, can be performed in classrooms using chalk to separate mixtures based on how strongly components adsorb to the chalk and dissolve in the solvent.
Original Description:
chalk Chromatography
Original Title
chalk Chromatography
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentChromatography is a technique used to separate mixtures into individual components. In 1906, Mikhail Tswett used calcium carbonate (chalk) to separate plant pigments by passing them through a chalk-filled glass tube with petroleum ether, resulting in distinct colored zones. This technique, known as column chromatography, can be performed in classrooms using chalk to separate mixtures based on how strongly components adsorb to the chalk and dissolve in the solvent.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
248 views1 pageChalk Chromatography
Chalk Chromatography
Uploaded by
Rojo JohnChromatography is a technique used to separate mixtures into individual components. In 1906, Mikhail Tswett used calcium carbonate (chalk) to separate plant pigments by passing them through a chalk-filled glass tube with petroleum ether, resulting in distinct colored zones. This technique, known as column chromatography, can be performed in classrooms using chalk to separate mixtures based on how strongly components adsorb to the chalk and dissolve in the solvent.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Chalk Chromatography
Chromatography is a technique used to separate components of a mixture.
In 1906, Mikhail Tswett, a Russian botanist, published a paper in which he
described the separation of pigments, extracted from green leaves, by washing
the mixture with petroleum ether (similar to lighter fluid) through a glass tube
packed with powdered calcium carbonate (chalk). As the mixture of pigments
passed down the CaCO3-filled tube, they separated into distinctly colored
zones. Tswett gave the name chromatography (the graphing of colors) to this
separation technique.
The method that Tswett used is known today as column chromatography.
The method of column chromatography can be carried out in the classroom
using calcium carbonate in the form of sticks of chalk. A mixture containing
two or more components is deposited on a stick of chalk, a solid adsorbing
substance. The components are adsorbed (i.e., held on the surface of the solid
substance) to varying degrees which depend on the nature of the component, the
nature of the adsorbent, and the temperature. Then the wash solvent (liquid) is
added to the adsorbent and allowed to flow through it by capillary effect. As the
solvent passes the deposited mixture, the components tend to be dissolved to
varying extents and are swept along the solid adsorbent.
The rate at which a component will move along the solid depends on its relative
tendency to be dissolved in the solvent and its tendency to be adsorbed on the
solid. The net effect is that, as the solvent passes slowly through the solid
adsorbent, the components of the mixture -separate from each other and move
along with the solvent forming rather diffuse zones or spots. With the proper
choice of solvent and adsorbent, it is possible to resolve many complex
mixtures into their components.
You might also like
- IGCSE Chemistry - States of Matter and Separation TechniquesDocument12 pagesIGCSE Chemistry - States of Matter and Separation TechniquesChemistryKlipz100% (24)
- ChromatographyDocument56 pagesChromatographyapi-26998277100% (14)
- Chromatography Is Used To Separate Mixtures of Substances Into Their ComponentsDocument8 pagesChromatography Is Used To Separate Mixtures of Substances Into Their ComponentsAyrea Riclye Sanaes'yumealoverNo ratings yet
- ChromatographyDocument68 pagesChromatographythamizh555No ratings yet
- ChromatographyDocument12 pagesChromatographyChristian MirandaNo ratings yet
- A. Title: Paper Chromatography B. Date: 28 C. Purpose: Determine RF On Markers Using Opaque Paper and MineralDocument4 pagesA. Title: Paper Chromatography B. Date: 28 C. Purpose: Determine RF On Markers Using Opaque Paper and MineralLailatul BadriyahNo ratings yet
- KromatografiDocument32 pagesKromatografiNofrizalNo ratings yet
- Column Chromatography, UV Visible, HPLCDocument6 pagesColumn Chromatography, UV Visible, HPLCnoman ahmadNo ratings yet
- ChromatographyDocument66 pagesChromatographyNidhal TrabelsiNo ratings yet
- States of Matter: Solids Have Particles That Are Packed Closely Together. The Atoms Are Arranged in ADocument92 pagesStates of Matter: Solids Have Particles That Are Packed Closely Together. The Atoms Are Arranged in AAminah ShahzadNo ratings yet
- Module 3 Part 2 Chromatographic TechniquesDocument11 pagesModule 3 Part 2 Chromatographic TechniquesJyolsna JayarajNo ratings yet
- Makalah KLTDocument9 pagesMakalah KLTretnofarahd5No ratings yet
- Thin Layer Chromatography and Column Chromatography: Activity No. 11Document9 pagesThin Layer Chromatography and Column Chromatography: Activity No. 11Mary Jean SteffenNo ratings yet
- Chemical Earth NotesDocument15 pagesChemical Earth NotesLuluNo ratings yet
- 222 Chapter 4Document35 pages222 Chapter 4talha saleemNo ratings yet
- Subdivision of Chromatography InstrumentDocument6 pagesSubdivision of Chromatography Instrumentmigom7md1No ratings yet
- Chromatographic TechniquesDocument42 pagesChromatographic TechniquesRaina JainNo ratings yet
- Unit-3 Adsorption and ChromatographyDocument51 pagesUnit-3 Adsorption and Chromatographyshiblast42 gamerNo ratings yet
- Exp 6 TLCDocument12 pagesExp 6 TLCGeorge PiliposyanNo ratings yet
- ASSIGNMENT Kundan DeoreDocument25 pagesASSIGNMENT Kundan DeoreKuNdAn DeOrENo ratings yet
- The Particulate Nature Of MatterDocument59 pagesThe Particulate Nature Of MatterKeyong SylvesterNo ratings yet
- 3-Liquid-Liquid Extraction+TLCDocument16 pages3-Liquid-Liquid Extraction+TLCLinh NguyễnNo ratings yet
- Histiria de La CromatografíaDocument9 pagesHistiria de La Cromatografíalagos_97No ratings yet
- 222 Chapter 4Document35 pages222 Chapter 4VengadeshNo ratings yet
- 1 - Basics of ChromatographyDocument37 pages1 - Basics of ChromatographyAnish KumarNo ratings yet
- Adsorption ChromatographyDocument4 pagesAdsorption ChromatographyIman FatimaNo ratings yet
- H?V Dgghwvfj8Xs: Classification of MatterDocument60 pagesH?V Dgghwvfj8Xs: Classification of MatterCheri BulahanNo ratings yet
- Principles of ChromatographyDocument5 pagesPrinciples of ChromatographyAnonymous v6cT39ENNo ratings yet
- CHROMATOGRAPHYDocument2 pagesCHROMATOGRAPHYMonica SreeNo ratings yet
- And Electrophoresis.: Solvent ExtractionDocument10 pagesAnd Electrophoresis.: Solvent Extractionyaseer786No ratings yet
- ChromatographyDocument38 pagesChromatographyelizabeth merzyNo ratings yet
- Chemistry Yr 9 Particle Reactions 2013Document28 pagesChemistry Yr 9 Particle Reactions 2013api-198270301No ratings yet
- Types of Chroma To Grap GyDocument75 pagesTypes of Chroma To Grap GyMohammad RehanNo ratings yet
- Mixture Separation Method Formative Task Nanda MarquesDocument3 pagesMixture Separation Method Formative Task Nanda Marquesmaria.marquesdamNo ratings yet
- Paper ChromatographyDocument6 pagesPaper ChromatographysamNo ratings yet
- Activity Sheet-25 (Separation of Mixtures)Document4 pagesActivity Sheet-25 (Separation of Mixtures)Nkemzi NzetengenleNo ratings yet
- Chap 1 NotesDocument9 pagesChap 1 NotesAe AeNo ratings yet
- Experimental Techniques (Chapter 2) : Presented By: Mrs. SaimaDocument16 pagesExperimental Techniques (Chapter 2) : Presented By: Mrs. SaimaMohamed MunirNo ratings yet
- Unit-4 Chromatography PDFDocument9 pagesUnit-4 Chromatography PDFhacker7899No ratings yet
- Igcse Complete Chemistry Notes: Unit 1: States of MatterDocument72 pagesIgcse Complete Chemistry Notes: Unit 1: States of MatterYoga RomdoniNo ratings yet
- Term Paper: BioanalyticalDocument17 pagesTerm Paper: BioanalyticalGyanendra GoshwamiNo ratings yet
- TLCDocument7 pagesTLCSnow DropNo ratings yet
- Solids Liquids and Gases: Solid PropertiesDocument42 pagesSolids Liquids and Gases: Solid PropertiesAmin Sharif SharifNo ratings yet
- Separating MixturesDocument6 pagesSeparating MixturesKylie AlmarezNo ratings yet
- Extraction Liquid - SolidDocument13 pagesExtraction Liquid - SolidHalkawt G MuhammadNo ratings yet
- ChromatographyDocument4 pagesChromatography湊崎エライザNo ratings yet
- IGCSE Complete Chemistry NotesDocument49 pagesIGCSE Complete Chemistry NotesUniba WajidNo ratings yet
- Study Guide Japan Part 1Document73 pagesStudy Guide Japan Part 1Janzu SalazarNo ratings yet
- Chem Reviewer FinalDocument13 pagesChem Reviewer Finalfelicity tejadaNo ratings yet
- Chromatography and Microscopy (BIO - 204)Document8 pagesChromatography and Microscopy (BIO - 204)Karen AgbaegbuNo ratings yet
- Analytical Techniques Final Note NADocument121 pagesAnalytical Techniques Final Note NAyuvi78312No ratings yet
- Chemistry Notes 2Document8 pagesChemistry Notes 2QfkjfksNo ratings yet
- Principle of ChromatographyDocument8 pagesPrinciple of ChromatographyMuhammad ZeeshanNo ratings yet
- Unit 3-ChromatographyDocument16 pagesUnit 3-ChromatographyAli SheikhNo ratings yet
- Module 2. Substaces and MixturesDocument19 pagesModule 2. Substaces and MixturesAshley Mae LumanasNo ratings yet
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksFrom EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo ratings yet
- O Level Biology Practice Questions And Answers Movement of substancesFrom EverandO Level Biology Practice Questions And Answers Movement of substancesNo ratings yet
- Coordination Compounds - BSC IIIDocument14 pagesCoordination Compounds - BSC IIIRojo John0% (1)
- PH.D Registration Information - 2019 (For Student Supervisor and Place of Research)Document5 pagesPH.D Registration Information - 2019 (For Student Supervisor and Place of Research)Rojo JohnNo ratings yet
- Kinetics of AtmosphereDocument4 pagesKinetics of AtmosphereRojo JohnNo ratings yet
- Kinetic Model CompletenessDocument5 pagesKinetic Model CompletenessMukund KsNo ratings yet
- Partial Molar PropertiesDocument5 pagesPartial Molar PropertiesRojo JohnNo ratings yet
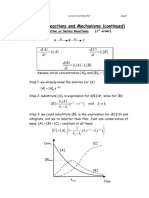
- Consecutive, Series, and Reversible ReactionsDocument6 pagesConsecutive, Series, and Reversible ReactionsRojo JohnNo ratings yet
- Two Component Phase Equilibria IDocument6 pagesTwo Component Phase Equilibria IRojo JohnNo ratings yet
- 3 - Rydberg Formula, Photoelectric Effect PDFDocument4 pages3 - Rydberg Formula, Photoelectric Effect PDFRojo JohnNo ratings yet
- Lecture 33: Normal Coordinate AnalysisDocument6 pagesLecture 33: Normal Coordinate AnalysisRojo JohnNo ratings yet
- Liqammonia PDFDocument7 pagesLiqammonia PDFRojo JohnNo ratings yet
- Multiply Bonded Metal-Ligand ComplexesDocument4 pagesMultiply Bonded Metal-Ligand ComplexesRojo JohnNo ratings yet
- 30 - Tanabe Sugano Diagrams PDFDocument5 pages30 - Tanabe Sugano Diagrams PDFRojo JohnNo ratings yet
- N-Dimensional Cyclic SystemDocument6 pagesN-Dimensional Cyclic SystemRojo JohnNo ratings yet
- Stereoselective and Asymmetric Synthesis PDFDocument12 pagesStereoselective and Asymmetric Synthesis PDFRojo JohnNo ratings yet
- Operator Properties&Mathematical GroupsDocument5 pagesOperator Properties&Mathematical GroupsRojo JohnNo ratings yet
- Molecular Point Group 1Document4 pagesMolecular Point Group 1Rojo JohnNo ratings yet
- Electronic Selection RulesDocument3 pagesElectronic Selection RulesRojo JohnNo ratings yet
- Spectrochemical SeriesDocument4 pagesSpectrochemical SeriesRojo JohnNo ratings yet