Professional Documents
Culture Documents
Genetics and Genomics Practical 2
Genetics and Genomics Practical 2
Uploaded by
MuhammadHuzaifahOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Genetics and Genomics Practical 2
Genetics and Genomics Practical 2
Uploaded by
MuhammadHuzaifahCopyright:
Available Formats
Analysis of Variation in the Human Genome using mtDNA,
amelogenin, Transposable element, VNTR, STR and RFLP
Primers
Muhammad Huzaifah bin Abd Rashid, Department of Biochemistry, Imperial College London, South
Kensington campus.
Here, we have a sample of DNA taken from a suspect extracted from a tissue found underneath
the fingernails in one of a murder victim and a set of DNA samples taken from the whole class.
Using some of the indispensable techniques in investigating the DNA samples such as Polymerase
Chain Reaction, Southern Blotting, DNA sequencing and analyzing variations at several different
loci, we can construct a genetic map to study the relationship between different populations or
individuals.
DNA Amplification and Gel Analysis Identities: 464/559 nucleotides match which
Using polymerase chain reaction (PCR), all the make it 84% similarities.
samples, including the suspect DNA are
amplified using different primers. The PCR Sample 3
products are then analyzed using gel
electrophoresis. Here are the results and Identities: 637/662 nucleotides match which
some discussions regarding the experiment make it 97% similarities.
using different type of primers.
Haplogroups analysis
1) Mitochondrial DNA (mtDNA)
From sample 1, from all the polymorphisms
Comparing three DNA sequences with the found, one substitution was found in position
suspect DNA: 16270 where C was substituted with T.
Sample 1 From sample 3, we found polymorphisms that
occur at position 16126 and 16294.
Identities: 639/662 nucleotides match which
make it 97% similarities. Unfortunately, for sample 2, the sequencing
quality was so bad; hence, there are too many
After analyzing the sequence, we can be sure unreliable sequencing data. Hence, we cannot
that there are real polymorphisms at certain determine confidently the haplogroup.
nucleotides especially with number 156, 203,
264. However, the difference in the ends of From the suspect DNA, we don’t have enough
the sequence may be due to poor quality such information to deduce the haplogroup.
as at nucleotides number 32, 635 and
onwards. Samples Haplogroup
1 UK
It clearly be seen that there are 2 Cannot be determined
polymorphisms in this sample compared to 3 T
the suspect. They are clearly not the same Suspect Cannot be determined
person.
2) Amelogenin
Sample 2
From the gel result, it is clearly seen two
bands from sample 1 and only one band from
sample 2 and 3. The suspect DNA forms two
bands on the gel electrophoresis.
The band with size around 433 bp amplified
from AMELX while the band with size 253 bp
is the product of AMELY amplification.
From the gel above, from sample 1, we can
As from the gel, we can conclude that sample clearly see a band with size about 424 bp. In
1 is a male while samples 2 and 3 are female. samples 2, 3 and the suspect, there is a band
The suspect is also a male, so, this rule out seen in the region of size about 113 bp.
both samples 2 and 3 from the list of suspects.
Hence, from the observations, we can deduce
From the gel, it can be seen that in sample 1, that individual 1 is homozygous for the
the larger band correspond to AMELX is insertion (+/+). Conversely, samples 2, 3 and
slightly faint compared to the other band. This the suspect are homozygous lacking the
might due to changes to the primer binding insertion (-/-).
site, hence, less primer binds and less
amplification occurs within that region. However, the result may be not quite reliable.
Larger fragments may difficult to amplify as
Amelogenin test is a good start in determining the Taq Polymerase has a low processivity.
the identity of the suspect. However, This means that this enzyme detach quickly
sometimes, the test can be quite misleading. before completing the elongation reaction.
For an example, if there are mutations in Y- Hence, there may be some larger fragments
derived fragments, this may result in failure of of other samples that fail to amplify to be
amplification of AMELY making the gender seen on the gel.
determination misleading.
Genotype Frequencies (class results):
In a scenario where the male and female Genotype Frequency
samples are mixed, we can mix the mixture +/+ 5/33
with cell lysis buffer, proteinase K, SDS and +/- 5/33
EDTA, then, centrifuge the mixture to -/- 23/33
separate the female sample as the
supernatant and the male fragment in the Allele Frequencies
pellet. Allele Frequency
+ 15/66
3) TPA25 (Transposable Element) - 51/66
Genotype Frequencies (expected)
Genotype Frequency
+/+ 2/44
+/- 12/44 Hence, it can be concluded that individual 1 is
-/- 30/44 heterozygous, while individual 3 is
homozygous for 21 repeats and the suspect is
The χ2 value for this set of data is 10.67013. homozygous for 24 repeats.
From this value, it can be concluded that the
genotype frequency of this population is not From the samples of the University of Utah,
consistent with Hardy-Weinberg Equilibrium. individual with the same D1S80 pattern as our
suspect are individual 6 and 10. However,
When the class results genotype frequency is there are possibilities that individual 21, 22,
compared with Asian genotype frequency, the 23 also share the same repeats due to slight
χ2 value calculated is 6.55316 which mean difference that may arise from the techniques
that the genotype distributions in both of the used. Other than these, all other samples can
populations are significantly different. be safely excluded.
4) D1S80 (Variable Number of Tandem The allele frequency of 24 repeats is 0.335 in
Repeats – VNTR) the UK. Hence, the probability of finding a
homozygous individual with 24 repeats is
(0.335)2 which is 0.112. The probability of
finding individual with the criteria is 0.0484 in
China.
5) Short Tandem Repeat – STR
20
The frequency of finding allele 164:
72
16
The frequency of finding allele 168:
72
20 16 5
Probability of finding a match: × =
72 72 81
From the above result, for each sample, we
can approximate the size of bands seen. The = 6.17 %
approximate sizes are recorded in this table:
6) DRD2 (Restriction Fragment Length
Sample Size of bands Number of Polymorphism – RFLP)
Repeats
1 550, 650 24, 31 Sample 1
2 No band -
3 500 21
Suspect 550 24
We can also see some faint bands of size
about 330 bp in (sample 1) and about 110 bp
(suspect). However, we do not consider these
as our bands of interest because their sizes
are not within the range that we expected
from the amplification. These bands might
occur due to non-specific amplifications.
There was no band seen from sample 2.
Sample 2 might have failed to amplify.
This shows that sample 2 are heterozygous for
these alleles. From the double digest, we can
clearly observe two smaller bands and one
Sample 2 band correspond to larger fragment. This
indicates that both restriction sites are on the
same chromosome.
In summary, we deduced these conditions:
Sample Genotype
1 T+B+/T+B+
2 T+B+/T-B-
3 T+B+/T+B+
Suspect T+B+/T+B+
The haplotype and allele frequencies from the
class this year is shown below:
Sample 3
Haplotyp Frequency Allele Frequency
e
+ +
TB 38/58 T+ 40/116
T-B- 17/58 T- 18/116
T+B- 2/58 B+ 39/116
T-B+ 1/58 B- 19/116
Linkage disequilibrium of SNPs:
38/58 – (40/116 x 39/116) = 0.539
= 53.9 % LD
Suspect DNA
From the gel from sample 1, we observed that
the entire samples are digested both in Taq1
and Bcl1, indicating that sample 1 is
homozygous for both of these sites. The same
observation can also be seen in sample 3 and
the suspect.
However, the digest from sample 2 is
different. It can clearly be seen 2 small bands
on the gel for both samples digested with
Taq1 and Bcl1. However, there are also a band
correspond to larger fragment on both digest.
You might also like
- Analyzing The PV92 Locus On Chromosome 16 For The Alu Insertion Through The Polymerase Chain Reaction and Agarose Gel ElectrophoresisDocument10 pagesAnalyzing The PV92 Locus On Chromosome 16 For The Alu Insertion Through The Polymerase Chain Reaction and Agarose Gel ElectrophoresisJohansen C. Pico100% (1)
- Castor Oil Packs ImmunomodulationDocument6 pagesCastor Oil Packs ImmunomodulationCiprian AlexandruNo ratings yet
- Chi Square TutorialDocument4 pagesChi Square TutorialCarina JLNo ratings yet
- Bio-Rad Crime Scene Student Pre-LabDocument5 pagesBio-Rad Crime Scene Student Pre-LabVictor YaoNo ratings yet
- (Analytical Profiles of Drug Substances 12) Klaus Florey (Eds.) - Academic Press (1983) PDFDocument736 pages(Analytical Profiles of Drug Substances 12) Klaus Florey (Eds.) - Academic Press (1983) PDFngochieu_909No ratings yet
- Kingdom Protista NotesDocument3 pagesKingdom Protista Noteshafizhusain0% (1)
- Medicine Cheat SheetsDocument16 pagesMedicine Cheat SheetsRisa Muthmainah100% (1)
- The Molecular Movement of The Polymerase Similar To Opening and Closing of The HandDocument23 pagesThe Molecular Movement of The Polymerase Similar To Opening and Closing of The HandIbna HayatiNo ratings yet
- DNA Finger PrintingDocument21 pagesDNA Finger PrintingMeylinda EnggiNo ratings yet
- Me TodosDocument17 pagesMe TodosAlejandro LoperaNo ratings yet
- Restriction Fragment Length PolymorphismDocument6 pagesRestriction Fragment Length PolymorphismAtul KumarNo ratings yet
- Important Numericals From ChaptersDocument10 pagesImportant Numericals From ChaptersSwastik DasNo ratings yet
- Genetics - Chapter 5 - Linked Gene InheritanceDocument46 pagesGenetics - Chapter 5 - Linked Gene InheritanceDuy AnhNo ratings yet
- Lingkages ProblemsDocument7 pagesLingkages ProblemsAnthony HugillNo ratings yet
- Genome Res. 2000 Germer 258 66Document10 pagesGenome Res. 2000 Germer 258 66Sambit Prasad KarNo ratings yet
- Statistical Analysis of Proportions: ExampleDocument42 pagesStatistical Analysis of Proportions: ExampleNadeem Altaf WahlaNo ratings yet
- Sensetivity of Karyotyping and MicroarrayDocument5 pagesSensetivity of Karyotyping and MicroarrayDr Ahmed Al AmriNo ratings yet
- Andy Cheng, Mike Garofalo, Jon Keung, Bo Li, Jenny Nguyen, Jeff WhartonDocument1 pageAndy Cheng, Mike Garofalo, Jon Keung, Bo Li, Jenny Nguyen, Jeff WhartonHafizah RamliNo ratings yet
- Paternity Testing New PDFDocument39 pagesPaternity Testing New PDFAnil Radhey100% (1)
- Forensic Science & DNA FingerprintingDocument4 pagesForensic Science & DNA FingerprintingWalwin HareNo ratings yet
- EXP F - Group 50 - Final VersionDocument5 pagesEXP F - Group 50 - Final VersionMarleen van der WielNo ratings yet
- Outline of Lecture 12 and 13Document3 pagesOutline of Lecture 12 and 13Efrain FloresNo ratings yet
- 2000 Rubin - Comparitive Genomics in EukaryotesDocument13 pages2000 Rubin - Comparitive Genomics in EukaryotesJenny ChenNo ratings yet
- Genetic Engineering 2 (T401) : DNA Analysis in Forensic Science & ArchaeologyDocument26 pagesGenetic Engineering 2 (T401) : DNA Analysis in Forensic Science & ArchaeologyMohamed MarghanyNo ratings yet
- LinkageMapping PresentationDocument25 pagesLinkageMapping PresentationAbaidullahNo ratings yet
- PCR DiscussionDocument3 pagesPCR DiscussionNadia Morales100% (2)
- DNA Profiling Student HandoutDocument9 pagesDNA Profiling Student HandoutNoah HolznerNo ratings yet
- Probes - RNA and DNA: Fluorescence in Situ Hybridization (FISH) Is ADocument4 pagesProbes - RNA and DNA: Fluorescence in Situ Hybridization (FISH) Is ACrina LupuNo ratings yet
- New Microsoft Office Word DocumentDocument20 pagesNew Microsoft Office Word DocumentSoumo PathakNo ratings yet
- Chromosome StructureDocument10 pagesChromosome StructurePhú NguyễnNo ratings yet
- DNA ProfilingDocument16 pagesDNA ProfilingSoundarya Chandramouleeswaran100% (1)
- Linkage MappingDocument5 pagesLinkage MappingDhakshayani GNo ratings yet
- Deoxyribonucleic Acid (DNA) Is A Large, Polymeric Molecule Found inDocument6 pagesDeoxyribonucleic Acid (DNA) Is A Large, Polymeric Molecule Found inTalitha NabilaNo ratings yet
- Sazci2012. Gen ZNF804ADocument5 pagesSazci2012. Gen ZNF804AFahrunnisa NurdinNo ratings yet
- Mol Bio in Paternity Testing & ForensicsDocument22 pagesMol Bio in Paternity Testing & ForensicsRainie PhamNo ratings yet
- Extremely High Levels of Human Mitochondrial DNA Heteroplasmy in Single Hair RootsDocument6 pagesExtremely High Levels of Human Mitochondrial DNA Heteroplasmy in Single Hair RootsBolje SutraNo ratings yet
- DNA Technology in Forensic SettingsDocument33 pagesDNA Technology in Forensic SettingsTAUZIAH SUFINo ratings yet
- Exon - IntronDocument4 pagesExon - IntronArJun ChandrasekharNo ratings yet
- Pre-Guía PCR (CSI Cali) BioQ SíDocument17 pagesPre-Guía PCR (CSI Cali) BioQ SíEsteban García TorresNo ratings yet
- WEEK 11 & 12 (Part 1) (CYTO)Document2 pagesWEEK 11 & 12 (Part 1) (CYTO)Hershei Vonne BaccayNo ratings yet
- Fish FTNWDocument8 pagesFish FTNWM.miracleNo ratings yet
- Predicting InheritanceDocument9 pagesPredicting InheritanceTadi MurombaNo ratings yet
- Primer To Population GeneticsDocument106 pagesPrimer To Population GeneticsΚατερίνα ΜέτσιουNo ratings yet
- DNA Profiling Activity STUDENTDocument8 pagesDNA Profiling Activity STUDENTMonalisa SahuNo ratings yet
- BioinformaticsDocument40 pagesBioinformaticsLazurdyNo ratings yet
- 红细胞血型血清学的实践和理论考虑2004Document4 pages红细胞血型血清学的实践和理论考虑2004kmbloodlzNo ratings yet
- Evidence of Linkage Between The Serotonin Transporter and Autistic DisorderDocument4 pagesEvidence of Linkage Between The Serotonin Transporter and Autistic DisorderMARGARITA HERNANDEZ MIXTECONo ratings yet
- 2016 Russo Prenat DiagnDocument6 pages2016 Russo Prenat DiagnClaudia CarcachaNo ratings yet
- Nucl. Acids Res. 2006 Le Caignec E68Document12 pagesNucl. Acids Res. 2006 Le Caignec E68mi2011saNo ratings yet
- DNA FingerprintingDocument20 pagesDNA Fingerprintingprayaasplb100% (1)
- Zhou Chromosome and Chromatin 1 NewDocument88 pagesZhou Chromosome and Chromatin 1 Newapi-3700537No ratings yet
- Ana Serafimovska FishDocument10 pagesAna Serafimovska FishtipharethtNo ratings yet
- A 21-Locus Autosomal SNP Multiplex and Its Application in Forensic ScienceDocument10 pagesA 21-Locus Autosomal SNP Multiplex and Its Application in Forensic ScienceVidini Kusuma AjiNo ratings yet
- Chapter 5 NotesDocument7 pagesChapter 5 NotesNandiniNo ratings yet
- Cytogenetics: Chromosome Banding & Barr Body DR Charu Khosla, 24/4/20, 10.45-11.45 AmDocument90 pagesCytogenetics: Chromosome Banding & Barr Body DR Charu Khosla, 24/4/20, 10.45-11.45 Amsonal aranha100% (2)
- Genetic Regulationof Nitrogen Fixationin RhizobiaDocument11 pagesGenetic Regulationof Nitrogen Fixationin RhizobiasakshiaroraissarNo ratings yet
- Microsatellite Allelic Homoplasy Due To Variable Flanking SequencesDocument5 pagesMicrosatellite Allelic Homoplasy Due To Variable Flanking SequencesVianey Sánchez FigueroaNo ratings yet
- Genetic Mapping & Recombination Frequency PDFDocument59 pagesGenetic Mapping & Recombination Frequency PDFNandiniNo ratings yet
- Name - Adhiraj Singh Chauhan Class - B.A.Llb Div. B' Year Iv' PRN - 14010125102 Subject - Forensic ScienceDocument5 pagesName - Adhiraj Singh Chauhan Class - B.A.Llb Div. B' Year Iv' PRN - 14010125102 Subject - Forensic ScienceAdhirajNo ratings yet
- Leukocyte Antigen (HLA)Document10 pagesLeukocyte Antigen (HLA)ALLISON PAMITTANNo ratings yet
- FISH Method of Chromosome PreparationDocument3 pagesFISH Method of Chromosome PreparationAppas SahaNo ratings yet
- LivakDocument24 pagesLivakibrahima1968No ratings yet
- Telomerases: Chemistry, Biology, and Clinical ApplicationsFrom EverandTelomerases: Chemistry, Biology, and Clinical ApplicationsNeal F. LueNo ratings yet
- JKMJKDocument5 pagesJKMJKSuci IrianiNo ratings yet
- MRCP Revision RheumatologyDocument35 pagesMRCP Revision RheumatologyLori BeckNo ratings yet
- Full Download Brock Biology of Microorganisms 13th Edition Madigan Test BankDocument35 pagesFull Download Brock Biology of Microorganisms 13th Edition Madigan Test Bankbeizatikeorar100% (23)
- ABO Subgroups and Bombay GroupDocument15 pagesABO Subgroups and Bombay GrouphamaadaNo ratings yet
- Augmentin DdsDocument11 pagesAugmentin DdsPrashanthBhatNo ratings yet
- Memorial Sloan Kettering Cancer Center Curriculum Vitae and BibliographyDocument6 pagesMemorial Sloan Kettering Cancer Center Curriculum Vitae and BibliographyOncoshravanNo ratings yet
- Cell Structure and FunctionDocument42 pagesCell Structure and FunctionRu RuNo ratings yet
- 26 Anti Dandruff ZMBMGSDocument7 pages26 Anti Dandruff ZMBMGSHarish BishtNo ratings yet
- Diuretics - : Five Major Classes of Diuretics AreDocument6 pagesDiuretics - : Five Major Classes of Diuretics AreVaishali PrasharNo ratings yet
- EL Husseinys Essentials of Biochemistry @eduwaves360Document260 pagesEL Husseinys Essentials of Biochemistry @eduwaves360Abdirahman abdallahNo ratings yet
- MTQ0OTY0ODkyNA PDFDocument2 pagesMTQ0OTY0ODkyNA PDFHansa BorichaNo ratings yet
- Dermatology An Illustrated Colour TextboDocument139 pagesDermatology An Illustrated Colour TextboHüseyin GündüzNo ratings yet
- Amoxicillin 125 MG 250 MG 5 ML Oral SuspensionDocument16 pagesAmoxicillin 125 MG 250 MG 5 ML Oral SuspensionAshrafNo ratings yet
- Answers For CH 11, 13, 14 Reading HandoutDocument19 pagesAnswers For CH 11, 13, 14 Reading HandoutJonathan DomenzainNo ratings yet
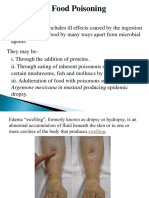
- Food Poisoning and IntoxicationsDocument26 pagesFood Poisoning and IntoxicationslakshmijayasriNo ratings yet
- Fish Immune Responses To Natural Infection With Carp Edema - 2022 - Fish - ShellDocument11 pagesFish Immune Responses To Natural Infection With Carp Edema - 2022 - Fish - Shellfredys seguraNo ratings yet
- Blood Bank Literature ReviewDocument5 pagesBlood Bank Literature Reviewbujuj1tunag2100% (1)
- PharmaceuticsDocument25 pagesPharmaceuticsvera GaborNo ratings yet
- Carcinoma GasterDocument32 pagesCarcinoma GasterAzhari AhsanNo ratings yet
- FUNGIDocument40 pagesFUNGIElla NajahNo ratings yet
- PMC The Personalized Medicine Report Opportunity Challenges and The FutureDocument72 pagesPMC The Personalized Medicine Report Opportunity Challenges and The Futuretanveer kaurNo ratings yet
- Evolution and Ecology of Plant VirusesDocument13 pagesEvolution and Ecology of Plant VirusesBarakNo ratings yet
- Caspase Detection AbCamDocument2 pagesCaspase Detection AbCamtacu tacuNo ratings yet
- Zika Virus PDFDocument2 pagesZika Virus PDFKrishna KumarNo ratings yet
- 10th ScienceDocument310 pages10th ScienceAbishek BachanNo ratings yet
- Clinical Practice Guidelines PSOHNS2016Document45 pagesClinical Practice Guidelines PSOHNS2016Jan Marvin Lichauco MendozaNo ratings yet