Professional Documents
Culture Documents
Dijagram Propan Butan Pritisak Pare Od Temperature
Dijagram Propan Butan Pritisak Pare Od Temperature
Uploaded by
AleksandarCopyright:
Available Formats
You might also like
- Internal Audit Checklist IMSDocument15 pagesInternal Audit Checklist IMSGREENEXE BUSINESS CONSULTANT100% (4)
- Manual Unity Pro PDFDocument612 pagesManual Unity Pro PDFThais Pontes0% (2)
- Propane Butane MixingDocument5 pagesPropane Butane Mixingkneller100% (1)
- Observation Table: BrassDocument5 pagesObservation Table: BrassAditya AnandNo ratings yet
- Chapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallDocument23 pagesChapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallselisenNo ratings yet
- Havells India LTD.: Pumpset Performance Test ReportDocument2 pagesHavells India LTD.: Pumpset Performance Test ReportAnonymous EnAgbHxNo ratings yet
- Zinc CuSO ExperimentDocument3 pagesZinc CuSO Experimentgeorgemarkoul100No ratings yet
- Remington Copper Hook-Up Wire Ampacity ChartsDocument1 pageRemington Copper Hook-Up Wire Ampacity ChartsAntonio AdorzaNo ratings yet
- Copper Nickel Metric Pipe StandardsDocument1 pageCopper Nickel Metric Pipe StandardsRahul JeganathanNo ratings yet
- Observation Table: SteelDocument3 pagesObservation Table: SteelAditya AnandNo ratings yet
- Laboratory of Unit Operations: Packed ColumnDocument18 pagesLaboratory of Unit Operations: Packed ColumnNguyễn ThuNo ratings yet
- Vapor Pressure of WaterDocument3 pagesVapor Pressure of WaterDương Hoàng100% (1)
- Fullerbetonprojete 0812 1Document1 pageFullerbetonprojete 0812 1Karim IderNo ratings yet
- Timbal Balik Fenol-Air: 2B PresentDocument8 pagesTimbal Balik Fenol-Air: 2B PresentElisabeth HapsariNo ratings yet
- Stage (M) Storage (M) Discharge (M /S) Stage (M) Storage (M) Dischargec (M /S)Document37 pagesStage (M) Storage (M) Discharge (M /S) Stage (M) Storage (M) Dischargec (M /S)kpop is moodNo ratings yet
- Curva de Titulacion PotenciometricaDocument3 pagesCurva de Titulacion PotenciometricaKatsumyNo ratings yet
- Elemental Analysis: Coal Tar Biomass TarDocument4 pagesElemental Analysis: Coal Tar Biomass TarCesar BerruecoNo ratings yet
- Spillway Flood Routine (Iwasaki)Document30 pagesSpillway Flood Routine (Iwasaki)johnstoneNo ratings yet
- R134a Pressure Temperature ChartDocument2 pagesR134a Pressure Temperature ChartHadeel AlrazimNo ratings yet
- LinearitasDocument5 pagesLinearitasAning 18No ratings yet
- Graph For Conversion Pressure Ba Cylinder 6L & 6.8LDocument7 pagesGraph For Conversion Pressure Ba Cylinder 6L & 6.8LFebriyanto Pandu PratamaNo ratings yet
- Cond Cooling Capacity, KW °C: Minimum Evaporating Temp. With: 10 K Suction Superheat Maximum Evaporating TemperatureDocument4 pagesCond Cooling Capacity, KW °C: Minimum Evaporating Temp. With: 10 K Suction Superheat Maximum Evaporating TemperaturemhafezsabanNo ratings yet
- Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Document42 pagesData Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Sri WahyuniNo ratings yet
- B11,12,13 (GRAFIK) 6janDocument52 pagesB11,12,13 (GRAFIK) 6janThoriq B. NusantaraNo ratings yet
- Ballmillfinish Mill Ball Charge CalculationDocument3 pagesBallmillfinish Mill Ball Charge Calculationfauzy2504No ratings yet
- Dia 1Document24 pagesDia 1Manuel SofmetNo ratings yet
- Partial Vapour Pressure of SO2 Over H2ODocument1 pagePartial Vapour Pressure of SO2 Over H2OJia Yuan ChngNo ratings yet
- YP154K1T-TFD - R32 - Data SheetDocument4 pagesYP154K1T-TFD - R32 - Data SheetSUYAMBU LINGAM ARUMUGAMNo ratings yet
- Wa0177Document6 pagesWa0177boris cedeñoNo ratings yet
- Dovelas EjercicioDocument15 pagesDovelas EjercicioJesus RedondoNo ratings yet
- Anilie Temp Vs Time Nitrobenzene Temp Vs TimeDocument8 pagesAnilie Temp Vs Time Nitrobenzene Temp Vs Timechintz BhatNo ratings yet
- Tiempo (Min) Peso (G) 1 Peso (G) 2 Promedio (G) X (G H2O/gMCS) WDocument3 pagesTiempo (Min) Peso (G) 1 Peso (G) 2 Promedio (G) X (G H2O/gMCS) WVicky MoralesNo ratings yet
- Analysis Evaluation Enzyme Action CatalaseDocument10 pagesAnalysis Evaluation Enzyme Action CatalaseStella CecchiniNo ratings yet
- LSFO Calculator: 2.5 FO System Capacity (Liters) 500 Date Time Position FO Consumption (M /HR)Document8 pagesLSFO Calculator: 2.5 FO System Capacity (Liters) 500 Date Time Position FO Consumption (M /HR)dharmawanNo ratings yet
- CHE3165 Practical Week 5 PDFDocument8 pagesCHE3165 Practical Week 5 PDFKhalid HassanNo ratings yet
- CHE3165 Practical Week 5 PDFDocument8 pagesCHE3165 Practical Week 5 PDFKhalid HassanNo ratings yet
- Fujitsu Troubleshooting Guide PDFDocument6 pagesFujitsu Troubleshooting Guide PDFИвайло ГайдарскиNo ratings yet
- Libro 1Document9 pagesLibro 1MENDOZA COLONIA CAMILO JOSENo ratings yet
- Binary System Thermo DyncamixDocument19 pagesBinary System Thermo DyncamixJohn Fritz FestejoNo ratings yet
- Mencari %mole Methanol UapDocument3 pagesMencari %mole Methanol UapChoiriah EBNo ratings yet
- EDM Surface Finish Charts 12-09.36214442Document2 pagesEDM Surface Finish Charts 12-09.36214442ical iculNo ratings yet
- 07 - Dew Point and RH Table PDFDocument3 pages07 - Dew Point and RH Table PDFvinod singhNo ratings yet
- Tarea 36 AssayDocument6 pagesTarea 36 AssayAlejo CastroNo ratings yet
- 50Hz Zp83Kce-Tfd R410A: Evaporating Temperature °CDocument2 pages50Hz Zp83Kce-Tfd R410A: Evaporating Temperature °CJesus GrilletNo ratings yet
- Azrlt15Z: Exxonmobil Refining and Supply Company 22777 Springwoods Village Parkway, Spring, TX 77389Document28 pagesAzrlt15Z: Exxonmobil Refining and Supply Company 22777 Springwoods Village Parkway, Spring, TX 77389Việt Anhd TrầnNo ratings yet
- Bangladesh University of Engineering and TechnologyDocument10 pagesBangladesh University of Engineering and TechnologyMd Abid AfridiNo ratings yet
- Supporting Information (Pages S1-S10) : Phosphorus Reclamation Through Hydrothermal Carbonization of Animal ManuresDocument9 pagesSupporting Information (Pages S1-S10) : Phosphorus Reclamation Through Hydrothermal Carbonization of Animal Manuresrtgersergtgr trghgrwthtrtehNo ratings yet
- VI. Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Document66 pagesVI. Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Kirana Wirr'sNo ratings yet
- Carta Psicometrica: Temperatura Bulbo Seco (°C)Document6 pagesCarta Psicometrica: Temperatura Bulbo Seco (°C)Sofía EscobarNo ratings yet
- Rodsim:: Open Circuit Grinding SimulatorDocument38 pagesRodsim:: Open Circuit Grinding SimulatorJunior Romero ChavezNo ratings yet
- 100W 5700kphotometric Test ReportDocument10 pages100W 5700kphotometric Test ReportGonzalo LunaNo ratings yet
- Fullerbetonprojete 0612Document6 pagesFullerbetonprojete 0612Karim IderNo ratings yet
- Fullerbetonprojete 0612Document6 pagesFullerbetonprojete 0612Karim IderNo ratings yet
- Auto RunDocument8 pagesAuto RunAhmed El HawariNo ratings yet
- D1 - D4 (Grafik)Document57 pagesD1 - D4 (Grafik)Thoriq B. NusantaraNo ratings yet
- Performance Test Results of Modified Bio-Asphalt.: Table 7Document1 pagePerformance Test Results of Modified Bio-Asphalt.: Table 7dazulu18No ratings yet
- Price List: Size Mass Length (M) (MM) (KG/LM) 6.0 7.5 9.0 10.5 12.0Document2 pagesPrice List: Size Mass Length (M) (MM) (KG/LM) 6.0 7.5 9.0 10.5 12.0MarkNeilDeeNo ratings yet
- LD3C 2 60W照度Document12 pagesLD3C 2 60W照度gorbytjokNo ratings yet
- Results and Discussions Heat Exp 3 KashDocument9 pagesResults and Discussions Heat Exp 3 KashMuhammad KasyfiNo ratings yet
- PPR Water Pipes and Fittings 20160719Document10 pagesPPR Water Pipes and Fittings 20160719tesemaNo ratings yet
- Hunter GastDocument1 pageHunter GastMarschall Nuñez GrandisonNo ratings yet
- EN12583 - 2007 PovucenDocument44 pagesEN12583 - 2007 PovucenAleksandarNo ratings yet
- Igem-Up-20 Introduction and ScopeDocument11 pagesIgem-Up-20 Introduction and ScopeAleksandarNo ratings yet
- IC GrejaciDocument353 pagesIC GrejaciAleksandarNo ratings yet
- Skripta - Prirucnik Za Polaganje Ispita Za Gas - 2009Document243 pagesSkripta - Prirucnik Za Polaganje Ispita Za Gas - 2009AleksandarNo ratings yet
- Geodetic Surveying of The Riverbed of Drin RiverDocument6 pagesGeodetic Surveying of The Riverbed of Drin RiverJohans BetiNo ratings yet
- Gen Guide Conc Segment AlDocument10 pagesGen Guide Conc Segment AlMarkNo ratings yet
- CSD - Foundational Skills Learning - HandbookDocument4 pagesCSD - Foundational Skills Learning - HandbookTeja RamNo ratings yet
- Eps Unit 3Document24 pagesEps Unit 3Bhavana PedadaNo ratings yet
- SL 718Document7 pagesSL 718Jessey StonerNo ratings yet
- Kaizen Report Quality and Safety Pocket Diary For Site Civil ActivityDocument16 pagesKaizen Report Quality and Safety Pocket Diary For Site Civil Activityshrirampandey99No ratings yet
- Test: Module 7 Vocabulary: PIONEER Beginners - American EditionDocument3 pagesTest: Module 7 Vocabulary: PIONEER Beginners - American EditionMiguel Angel Alvarado50% (2)
- Chapter 17-18 Conflict, Negotiation, and TechnologyDocument72 pagesChapter 17-18 Conflict, Negotiation, and TechnologyHaryadi WidodoNo ratings yet
- Arduino - How To Control Servo Motor With Potentiometer - 5 Steps (With Pictures) - InstructablesDocument7 pagesArduino - How To Control Servo Motor With Potentiometer - 5 Steps (With Pictures) - InstructablesboucharebNo ratings yet
- GettingStartedenUS en USDocument242 pagesGettingStartedenUS en USKadir BoyluNo ratings yet
- Calculatecylinder HeadsDocument23 pagesCalculatecylinder HeadsAwingNo ratings yet
- Gas Storage-Main PresentationDocument21 pagesGas Storage-Main PresentationMurad RustamliNo ratings yet
- Q Series-1Document4 pagesQ Series-1williams vasquezNo ratings yet
- Scale of Data MeasurementDocument11 pagesScale of Data MeasurementSaudulla Jameel JameelNo ratings yet
- PDFDocument252 pagesPDFWael BazziNo ratings yet
- Bulk Flow - Micro/ultrafiltrationDocument5 pagesBulk Flow - Micro/ultrafiltrationjaNo ratings yet
- Assistant S & GAD 2018Document9 pagesAssistant S & GAD 2018Awais YounasNo ratings yet
- Private Vihicles POF Etag PDFDocument2 pagesPrivate Vihicles POF Etag PDFGM B&GNo ratings yet
- Trial and Activation InstructionsDocument18 pagesTrial and Activation InstructionsLuis LabradaNo ratings yet
- Effectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicDocument5 pagesEffectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicBala BhaskarNo ratings yet
- DAA Mid Term Assessment (Spring-2020, Semester) : Muhammad Arif Bahria University Lahore CampusDocument4 pagesDAA Mid Term Assessment (Spring-2020, Semester) : Muhammad Arif Bahria University Lahore CampusHamza Phanoti0% (1)
- About 5paisa PDFDocument2 pagesAbout 5paisa PDFShashwat ShrivastavaNo ratings yet
- PBL 5 - Example of Real-World Use of Computer Forensics and Cyber Crime AnswerDocument3 pagesPBL 5 - Example of Real-World Use of Computer Forensics and Cyber Crime AnswerBinaya ShresthaNo ratings yet
- For Education Purpose: International StandardDocument40 pagesFor Education Purpose: International StandardChristianieAnn100% (1)
- BH LearnDocument3 pagesBH LearnFARDEEN AHMED 18BCY10034No ratings yet
- Perryware Master CatalogDocument85 pagesPerryware Master Catalogsidharthpa7756No ratings yet
- Influocial Digital Portfolio-2021Document11 pagesInfluocial Digital Portfolio-2021Influocial Technologies Pvt Ltd100% (1)
Dijagram Propan Butan Pritisak Pare Od Temperature
Dijagram Propan Butan Pritisak Pare Od Temperature
Uploaded by
AleksandarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dijagram Propan Butan Pritisak Pare Od Temperature
Dijagram Propan Butan Pritisak Pare Od Temperature
Uploaded by
AleksandarCopyright:
Available Formats
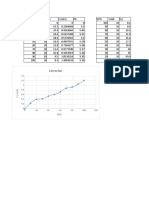
Propane is more suited to colder environments since it evaporates at -44oF (-42oC) at
atmospheric pressure. Butane evaporates at 33oF (0.6 oC) at atmospheric pressure.
The vapor pressure of a mixture of the two products are indicated in the table below:
Vapor Pressure (psig)
Propane
(C3H8) 100 70 50 30 0
(%)
Mixture
Butane
(C4H10) 0 30 50 70 100
(%)
-44 0 0 0 0 0
-30 6.8 0 0 0 0
-20 11.5 4.7 0 0 0
-10 17.5 9 3.5 0 0
0 24.5 15 7.6 2.3 0
10 34 20.5 12.3 5.9 0
20 42 28 17.8 10.2 0
Temperature 30 53 36.5 24.5 15.4 0
(oF) 40 65 46 32.4 21.5 3.1
50 78 56 41 28.5 6.9
60 93 68 50 36.5 11.5
70 110 82 61 45 17
80 128 96 74 54 23
90 150 114 88 66 30
100 177 134 104 79 38
110 204 158 122 93 47
1 psi (lb/in2) = 144 psf (lbf/ft2) = 6,894.8 Pa (N/m2) = 6.895x10-3 N/mm2 = 6.895x10-2 bar
Note that the evaporation temperature is not the only parameter that influences on evaporation of
the propane butane mixture. Evaporation requires heat and if the heat transferred to the liquid gas
is limited - the liquid cools down and the evaporation is reduced.
Larger consumers requires heat exchangers fueled with hot water, electric heater or
combustion of the propane butane mix itself to supply the evaporation heat.
Smaller consumers requires propane butane mix containers with efficient heat transfer
from the surroundings. By example - composite containers provides less heat transfer
compared to steel containers and may cause problems at lower surroundings
temperatures.
Propane Butane Mix Vapor Diagram - psig
Propane Butane Mix Vapor Diagram - bar
You might also like
- Internal Audit Checklist IMSDocument15 pagesInternal Audit Checklist IMSGREENEXE BUSINESS CONSULTANT100% (4)
- Manual Unity Pro PDFDocument612 pagesManual Unity Pro PDFThais Pontes0% (2)
- Propane Butane MixingDocument5 pagesPropane Butane Mixingkneller100% (1)
- Observation Table: BrassDocument5 pagesObservation Table: BrassAditya AnandNo ratings yet
- Chapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallDocument23 pagesChapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallselisenNo ratings yet
- Havells India LTD.: Pumpset Performance Test ReportDocument2 pagesHavells India LTD.: Pumpset Performance Test ReportAnonymous EnAgbHxNo ratings yet
- Zinc CuSO ExperimentDocument3 pagesZinc CuSO Experimentgeorgemarkoul100No ratings yet
- Remington Copper Hook-Up Wire Ampacity ChartsDocument1 pageRemington Copper Hook-Up Wire Ampacity ChartsAntonio AdorzaNo ratings yet
- Copper Nickel Metric Pipe StandardsDocument1 pageCopper Nickel Metric Pipe StandardsRahul JeganathanNo ratings yet
- Observation Table: SteelDocument3 pagesObservation Table: SteelAditya AnandNo ratings yet
- Laboratory of Unit Operations: Packed ColumnDocument18 pagesLaboratory of Unit Operations: Packed ColumnNguyễn ThuNo ratings yet
- Vapor Pressure of WaterDocument3 pagesVapor Pressure of WaterDương Hoàng100% (1)
- Fullerbetonprojete 0812 1Document1 pageFullerbetonprojete 0812 1Karim IderNo ratings yet
- Timbal Balik Fenol-Air: 2B PresentDocument8 pagesTimbal Balik Fenol-Air: 2B PresentElisabeth HapsariNo ratings yet
- Stage (M) Storage (M) Discharge (M /S) Stage (M) Storage (M) Dischargec (M /S)Document37 pagesStage (M) Storage (M) Discharge (M /S) Stage (M) Storage (M) Dischargec (M /S)kpop is moodNo ratings yet
- Curva de Titulacion PotenciometricaDocument3 pagesCurva de Titulacion PotenciometricaKatsumyNo ratings yet
- Elemental Analysis: Coal Tar Biomass TarDocument4 pagesElemental Analysis: Coal Tar Biomass TarCesar BerruecoNo ratings yet
- Spillway Flood Routine (Iwasaki)Document30 pagesSpillway Flood Routine (Iwasaki)johnstoneNo ratings yet
- R134a Pressure Temperature ChartDocument2 pagesR134a Pressure Temperature ChartHadeel AlrazimNo ratings yet
- LinearitasDocument5 pagesLinearitasAning 18No ratings yet
- Graph For Conversion Pressure Ba Cylinder 6L & 6.8LDocument7 pagesGraph For Conversion Pressure Ba Cylinder 6L & 6.8LFebriyanto Pandu PratamaNo ratings yet
- Cond Cooling Capacity, KW °C: Minimum Evaporating Temp. With: 10 K Suction Superheat Maximum Evaporating TemperatureDocument4 pagesCond Cooling Capacity, KW °C: Minimum Evaporating Temp. With: 10 K Suction Superheat Maximum Evaporating TemperaturemhafezsabanNo ratings yet
- Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Document42 pagesData Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Sri WahyuniNo ratings yet
- B11,12,13 (GRAFIK) 6janDocument52 pagesB11,12,13 (GRAFIK) 6janThoriq B. NusantaraNo ratings yet
- Ballmillfinish Mill Ball Charge CalculationDocument3 pagesBallmillfinish Mill Ball Charge Calculationfauzy2504No ratings yet
- Dia 1Document24 pagesDia 1Manuel SofmetNo ratings yet
- Partial Vapour Pressure of SO2 Over H2ODocument1 pagePartial Vapour Pressure of SO2 Over H2OJia Yuan ChngNo ratings yet
- YP154K1T-TFD - R32 - Data SheetDocument4 pagesYP154K1T-TFD - R32 - Data SheetSUYAMBU LINGAM ARUMUGAMNo ratings yet
- Wa0177Document6 pagesWa0177boris cedeñoNo ratings yet
- Dovelas EjercicioDocument15 pagesDovelas EjercicioJesus RedondoNo ratings yet
- Anilie Temp Vs Time Nitrobenzene Temp Vs TimeDocument8 pagesAnilie Temp Vs Time Nitrobenzene Temp Vs Timechintz BhatNo ratings yet
- Tiempo (Min) Peso (G) 1 Peso (G) 2 Promedio (G) X (G H2O/gMCS) WDocument3 pagesTiempo (Min) Peso (G) 1 Peso (G) 2 Promedio (G) X (G H2O/gMCS) WVicky MoralesNo ratings yet
- Analysis Evaluation Enzyme Action CatalaseDocument10 pagesAnalysis Evaluation Enzyme Action CatalaseStella CecchiniNo ratings yet
- LSFO Calculator: 2.5 FO System Capacity (Liters) 500 Date Time Position FO Consumption (M /HR)Document8 pagesLSFO Calculator: 2.5 FO System Capacity (Liters) 500 Date Time Position FO Consumption (M /HR)dharmawanNo ratings yet
- CHE3165 Practical Week 5 PDFDocument8 pagesCHE3165 Practical Week 5 PDFKhalid HassanNo ratings yet
- CHE3165 Practical Week 5 PDFDocument8 pagesCHE3165 Practical Week 5 PDFKhalid HassanNo ratings yet
- Fujitsu Troubleshooting Guide PDFDocument6 pagesFujitsu Troubleshooting Guide PDFИвайло ГайдарскиNo ratings yet
- Libro 1Document9 pagesLibro 1MENDOZA COLONIA CAMILO JOSENo ratings yet
- Binary System Thermo DyncamixDocument19 pagesBinary System Thermo DyncamixJohn Fritz FestejoNo ratings yet
- Mencari %mole Methanol UapDocument3 pagesMencari %mole Methanol UapChoiriah EBNo ratings yet
- EDM Surface Finish Charts 12-09.36214442Document2 pagesEDM Surface Finish Charts 12-09.36214442ical iculNo ratings yet
- 07 - Dew Point and RH Table PDFDocument3 pages07 - Dew Point and RH Table PDFvinod singhNo ratings yet
- Tarea 36 AssayDocument6 pagesTarea 36 AssayAlejo CastroNo ratings yet
- 50Hz Zp83Kce-Tfd R410A: Evaporating Temperature °CDocument2 pages50Hz Zp83Kce-Tfd R410A: Evaporating Temperature °CJesus GrilletNo ratings yet
- Azrlt15Z: Exxonmobil Refining and Supply Company 22777 Springwoods Village Parkway, Spring, TX 77389Document28 pagesAzrlt15Z: Exxonmobil Refining and Supply Company 22777 Springwoods Village Parkway, Spring, TX 77389Việt Anhd TrầnNo ratings yet
- Bangladesh University of Engineering and TechnologyDocument10 pagesBangladesh University of Engineering and TechnologyMd Abid AfridiNo ratings yet
- Supporting Information (Pages S1-S10) : Phosphorus Reclamation Through Hydrothermal Carbonization of Animal ManuresDocument9 pagesSupporting Information (Pages S1-S10) : Phosphorus Reclamation Through Hydrothermal Carbonization of Animal Manuresrtgersergtgr trghgrwthtrtehNo ratings yet
- VI. Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Document66 pagesVI. Data Pengamatan 1. Manual: Hubungan PSV Dengan F1 Hot Pump Setting: 50%Kirana Wirr'sNo ratings yet
- Carta Psicometrica: Temperatura Bulbo Seco (°C)Document6 pagesCarta Psicometrica: Temperatura Bulbo Seco (°C)Sofía EscobarNo ratings yet
- Rodsim:: Open Circuit Grinding SimulatorDocument38 pagesRodsim:: Open Circuit Grinding SimulatorJunior Romero ChavezNo ratings yet
- 100W 5700kphotometric Test ReportDocument10 pages100W 5700kphotometric Test ReportGonzalo LunaNo ratings yet
- Fullerbetonprojete 0612Document6 pagesFullerbetonprojete 0612Karim IderNo ratings yet
- Fullerbetonprojete 0612Document6 pagesFullerbetonprojete 0612Karim IderNo ratings yet
- Auto RunDocument8 pagesAuto RunAhmed El HawariNo ratings yet
- D1 - D4 (Grafik)Document57 pagesD1 - D4 (Grafik)Thoriq B. NusantaraNo ratings yet
- Performance Test Results of Modified Bio-Asphalt.: Table 7Document1 pagePerformance Test Results of Modified Bio-Asphalt.: Table 7dazulu18No ratings yet
- Price List: Size Mass Length (M) (MM) (KG/LM) 6.0 7.5 9.0 10.5 12.0Document2 pagesPrice List: Size Mass Length (M) (MM) (KG/LM) 6.0 7.5 9.0 10.5 12.0MarkNeilDeeNo ratings yet
- LD3C 2 60W照度Document12 pagesLD3C 2 60W照度gorbytjokNo ratings yet
- Results and Discussions Heat Exp 3 KashDocument9 pagesResults and Discussions Heat Exp 3 KashMuhammad KasyfiNo ratings yet
- PPR Water Pipes and Fittings 20160719Document10 pagesPPR Water Pipes and Fittings 20160719tesemaNo ratings yet
- Hunter GastDocument1 pageHunter GastMarschall Nuñez GrandisonNo ratings yet
- EN12583 - 2007 PovucenDocument44 pagesEN12583 - 2007 PovucenAleksandarNo ratings yet
- Igem-Up-20 Introduction and ScopeDocument11 pagesIgem-Up-20 Introduction and ScopeAleksandarNo ratings yet
- IC GrejaciDocument353 pagesIC GrejaciAleksandarNo ratings yet
- Skripta - Prirucnik Za Polaganje Ispita Za Gas - 2009Document243 pagesSkripta - Prirucnik Za Polaganje Ispita Za Gas - 2009AleksandarNo ratings yet
- Geodetic Surveying of The Riverbed of Drin RiverDocument6 pagesGeodetic Surveying of The Riverbed of Drin RiverJohans BetiNo ratings yet
- Gen Guide Conc Segment AlDocument10 pagesGen Guide Conc Segment AlMarkNo ratings yet
- CSD - Foundational Skills Learning - HandbookDocument4 pagesCSD - Foundational Skills Learning - HandbookTeja RamNo ratings yet
- Eps Unit 3Document24 pagesEps Unit 3Bhavana PedadaNo ratings yet
- SL 718Document7 pagesSL 718Jessey StonerNo ratings yet
- Kaizen Report Quality and Safety Pocket Diary For Site Civil ActivityDocument16 pagesKaizen Report Quality and Safety Pocket Diary For Site Civil Activityshrirampandey99No ratings yet
- Test: Module 7 Vocabulary: PIONEER Beginners - American EditionDocument3 pagesTest: Module 7 Vocabulary: PIONEER Beginners - American EditionMiguel Angel Alvarado50% (2)
- Chapter 17-18 Conflict, Negotiation, and TechnologyDocument72 pagesChapter 17-18 Conflict, Negotiation, and TechnologyHaryadi WidodoNo ratings yet
- Arduino - How To Control Servo Motor With Potentiometer - 5 Steps (With Pictures) - InstructablesDocument7 pagesArduino - How To Control Servo Motor With Potentiometer - 5 Steps (With Pictures) - InstructablesboucharebNo ratings yet
- GettingStartedenUS en USDocument242 pagesGettingStartedenUS en USKadir BoyluNo ratings yet
- Calculatecylinder HeadsDocument23 pagesCalculatecylinder HeadsAwingNo ratings yet
- Gas Storage-Main PresentationDocument21 pagesGas Storage-Main PresentationMurad RustamliNo ratings yet
- Q Series-1Document4 pagesQ Series-1williams vasquezNo ratings yet
- Scale of Data MeasurementDocument11 pagesScale of Data MeasurementSaudulla Jameel JameelNo ratings yet
- PDFDocument252 pagesPDFWael BazziNo ratings yet
- Bulk Flow - Micro/ultrafiltrationDocument5 pagesBulk Flow - Micro/ultrafiltrationjaNo ratings yet
- Assistant S & GAD 2018Document9 pagesAssistant S & GAD 2018Awais YounasNo ratings yet
- Private Vihicles POF Etag PDFDocument2 pagesPrivate Vihicles POF Etag PDFGM B&GNo ratings yet
- Trial and Activation InstructionsDocument18 pagesTrial and Activation InstructionsLuis LabradaNo ratings yet
- Effectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicDocument5 pagesEffectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicBala BhaskarNo ratings yet
- DAA Mid Term Assessment (Spring-2020, Semester) : Muhammad Arif Bahria University Lahore CampusDocument4 pagesDAA Mid Term Assessment (Spring-2020, Semester) : Muhammad Arif Bahria University Lahore CampusHamza Phanoti0% (1)
- About 5paisa PDFDocument2 pagesAbout 5paisa PDFShashwat ShrivastavaNo ratings yet
- PBL 5 - Example of Real-World Use of Computer Forensics and Cyber Crime AnswerDocument3 pagesPBL 5 - Example of Real-World Use of Computer Forensics and Cyber Crime AnswerBinaya ShresthaNo ratings yet
- For Education Purpose: International StandardDocument40 pagesFor Education Purpose: International StandardChristianieAnn100% (1)
- BH LearnDocument3 pagesBH LearnFARDEEN AHMED 18BCY10034No ratings yet
- Perryware Master CatalogDocument85 pagesPerryware Master Catalogsidharthpa7756No ratings yet
- Influocial Digital Portfolio-2021Document11 pagesInfluocial Digital Portfolio-2021Influocial Technologies Pvt Ltd100% (1)