Professional Documents
Culture Documents
Thermogravimetric Analysis of Calcium Oxalate
Thermogravimetric Analysis of Calcium Oxalate
Uploaded by
akash babelCopyright:
Available Formats
You might also like
- Chemical Equilibria Tutorial With AnsDocument6 pagesChemical Equilibria Tutorial With AnsDomNo ratings yet
- Free Fall Colla'seDocument3 pagesFree Fall Colla'sesendra20No ratings yet
- HW#3 Optoelectronics 109ADocument2 pagesHW#3 Optoelectronics 109ALuo Mark100% (1)
- Contoh Soal Dan Penyelesain Ke2Document4 pagesContoh Soal Dan Penyelesain Ke2Siti HannaNo ratings yet
- Chemical EquilibriumDocument18 pagesChemical EquilibriumCarbuncle JonesNo ratings yet
- PE - Online SKOLTECH PDFDocument14 pagesPE - Online SKOLTECH PDFOscar100% (1)
- HW 8Document2 pagesHW 8Hai Pham0% (1)
- Emcf 5 F14Document4 pagesEmcf 5 F14ShitPre SuperNo ratings yet
- Sol Ch5 Part2Document5 pagesSol Ch5 Part2mazharNo ratings yet
- Chem 11 Final Exam Review KeyDocument12 pagesChem 11 Final Exam Review Keyboriana72No ratings yet
- Process Dynamics and Control, Ch. 24 Solution ManualDocument27 pagesProcess Dynamics and Control, Ch. 24 Solution ManualBen Spearman100% (1)
- Solution Manual PDFDocument162 pagesSolution Manual PDFBerihu GirmayNo ratings yet
- Final Exam 2012Document12 pagesFinal Exam 2012Mat MorashNo ratings yet
- Earthing Design CalculationsDocument7 pagesEarthing Design CalculationsVamsiNo ratings yet
- ASTM C-40 Tester: Ordering InformationDocument1 pageASTM C-40 Tester: Ordering InformationImber Rene Salgueiro HuancaNo ratings yet
- Engr-2500u Midterm SolutionsDocument6 pagesEngr-2500u Midterm SolutionsAbdullah AlshihriNo ratings yet
- Reactors 2 SolutionsDocument4 pagesReactors 2 Solutionsnripesh_pokhrel100% (2)
- CH 5 PT 1Document3 pagesCH 5 PT 1Jennifer Chang100% (1)
- Solutions Hints 2Document6 pagesSolutions Hints 2iftikhar4498929No ratings yet
- Set6ans 10Document4 pagesSet6ans 10Natália FerreiraNo ratings yet
- Problem Set 3Document3 pagesProblem Set 3AshutoshKumarNo ratings yet
- AIEEE - 2011 Paper With Solutions For Physics, Chemistry and MathsDocument16 pagesAIEEE - 2011 Paper With Solutions For Physics, Chemistry and Mathsstudysteps.inNo ratings yet
- Solutions ManualDocument17 pagesSolutions ManualTzu-li LiuNo ratings yet
- Homework Solutions Lamarsh Chap 5Document10 pagesHomework Solutions Lamarsh Chap 5MarcoMoraNo ratings yet
- 2018 H2 Chemistry Paper 4 (Ans)Document8 pages2018 H2 Chemistry Paper 4 (Ans)Justin GohNo ratings yet
- Code 0 p2 SolutionDocument38 pagesCode 0 p2 Solutionanon020202No ratings yet
- Natural Convection Flow Over Flat Plate Integral SolutionDocument11 pagesNatural Convection Flow Over Flat Plate Integral Solutionsandyengineer13No ratings yet
- Lamarsh ErrataDocument2 pagesLamarsh ErrataMuhammad AbdullahNo ratings yet
- Assignment 1 - Chapter 3 AnswerDocument9 pagesAssignment 1 - Chapter 3 AnswerHarryzam MartelNo ratings yet
- STA 247 - Answers For Practice Problem Set #1Document5 pagesSTA 247 - Answers For Practice Problem Set #1aakasNo ratings yet
- ACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1Document10 pagesACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1ofji4oNo ratings yet
- CHEM1070B - Assignment 3 KeyDocument5 pagesCHEM1070B - Assignment 3 Keymakabigail7No ratings yet
- ch4-6 SlipDocument5 pagesch4-6 SlipAyesha Farooq100% (1)
- Engineering Materials 2 - Chapter 18Document4 pagesEngineering Materials 2 - Chapter 18Andres PerezNo ratings yet
- ENGRD 221 - Thermodynamics (Prof. N. Zabaras) Prelim IDocument9 pagesENGRD 221 - Thermodynamics (Prof. N. Zabaras) Prelim IMurat TülekNo ratings yet
- Modelling Reactors Kinetics PDFDocument65 pagesModelling Reactors Kinetics PDFSachini SarthchandraNo ratings yet
- Chapter 3 OxidationDocument49 pagesChapter 3 OxidationsunNo ratings yet
- Problem Set 1 SolutionsDocument4 pagesProblem Set 1 SolutionsAnshu Kumar GuptaNo ratings yet
- Problem 1. A conducting slab: I ikz−ωtDocument38 pagesProblem 1. A conducting slab: I ikz−ωtHaroonRashidNo ratings yet
- Workbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsDocument14 pagesWorkbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsShubham mishraNo ratings yet
- Problems SetDocument10 pagesProblems SetSajith KurianNo ratings yet
- Corrosion Assignment-2018Document2 pagesCorrosion Assignment-2018Huy An Mai0% (2)
- Peak Position PDFDocument56 pagesPeak Position PDFkumar ujjwalNo ratings yet
- Rate Expression and Activation Energy: 1a. (1 Mark)Document7 pagesRate Expression and Activation Energy: 1a. (1 Mark)senna0% (1)
- CHM3010 Module Thermodynamic-AnsDocument2 pagesCHM3010 Module Thermodynamic-Ansnur hashimahNo ratings yet
- 2006 JC 1 H2 JCT & Promo - Differential EquationsDocument3 pages2006 JC 1 H2 JCT & Promo - Differential EquationsOccamsRazorNo ratings yet
- ch4 ExamplesDocument11 pagesch4 ExamplesSary KilanyNo ratings yet
- ch19 PDFDocument24 pagesch19 PDFRodrigo S QuirinoNo ratings yet
- Tarea 2 FisicaDocument5 pagesTarea 2 FisicaNestor UlloaNo ratings yet
- Worksheet 2Document5 pagesWorksheet 2Theødřøš ÄbNo ratings yet
- 849sample PDFDocument16 pages849sample PDFAryan AsharNo ratings yet
- AJC H2CHEM 2007 Prelims Paper 1Document15 pagesAJC H2CHEM 2007 Prelims Paper 1chuasioklengNo ratings yet
- Resolução Exercs. Cienc. Dos MateriaisDocument19 pagesResolução Exercs. Cienc. Dos MateriaisRafael AraújoNo ratings yet
- Vakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6Document15 pagesVakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6vigiraneza0No ratings yet
- Chapter 7 Diffusion in SolidsDocument39 pagesChapter 7 Diffusion in SolidsNayak Swaraj PankajNo ratings yet
- Transport Processes in The Environment 5Document23 pagesTransport Processes in The Environment 5Amit KumarNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Atomic Absorption Spectroscopy: International Atomic Absorption Spectroscopy ConferenceFrom EverandAtomic Absorption Spectroscopy: International Atomic Absorption Spectroscopy ConferenceR. M. DagnallNo ratings yet
- Corrosion Inhibition Performance of Copper Carbonate in MEADocument5 pagesCorrosion Inhibition Performance of Copper Carbonate in MEAdow2008No ratings yet
- Physical/ Chemical Changes: CGA #2 ReviewDocument8 pagesPhysical/ Chemical Changes: CGA #2 Reviewengchemistry18No ratings yet
- Eugenio Ochoa GonzalezDocument3 pagesEugenio Ochoa GonzalezEugenio Ochoa GonzalezNo ratings yet
- GloverDocument272 pagesGlovermidialaoropesaNo ratings yet
- MB0052-Strategic Management and Business Policy (Assignment-1)Document9 pagesMB0052-Strategic Management and Business Policy (Assignment-1)Anil KumarNo ratings yet
- Villa As ParadigmDocument4 pagesVilla As ParadigmPaul Turner100% (1)
- Push Button Typical WiringDocument12 pagesPush Button Typical Wiringstrob1974No ratings yet
- Indirani College of Nursing: Level of Student - B.SC (N) Ii Yrs TractionDocument7 pagesIndirani College of Nursing: Level of Student - B.SC (N) Ii Yrs TractiondhanasundariNo ratings yet
- MR - Tushar Thombare Resume PDFDocument3 pagesMR - Tushar Thombare Resume PDFprasadNo ratings yet
- Jak Na Power Bi Cheat SheetDocument3 pagesJak Na Power Bi Cheat SheetenggdkteNo ratings yet
- Trade WorksheetDocument11 pagesTrade WorksheetAsma KamranNo ratings yet
- Unit 1 - 18EC61Document93 pagesUnit 1 - 18EC61Pritam SarkarNo ratings yet
- Mathematics Grade 10Document270 pagesMathematics Grade 10TammanurRaviNo ratings yet
- Tugas Uas Miss LennyDocument8 pagesTugas Uas Miss LennyZiyan Khoirun hakimNo ratings yet
- Genesis g16Document2 pagesGenesis g16Krist UtamaNo ratings yet
- Standard Electrode Potentials & CellsDocument3 pagesStandard Electrode Potentials & Cellsmy nameNo ratings yet
- Control System Kec 602Document2 pagesControl System Kec 602Nitya MishraNo ratings yet
- 128M (8Mx16) GDDR SDRAM: HY5DU281622ETDocument34 pages128M (8Mx16) GDDR SDRAM: HY5DU281622ETBoris LazarchukNo ratings yet
- Adobe Photoshop CS6 Exam SimulationDocument6 pagesAdobe Photoshop CS6 Exam SimulationMuhammad NadhirNo ratings yet
- EV-100ZX Motor Controller Description & RepairDocument95 pagesEV-100ZX Motor Controller Description & RepairMario Aguirre100% (1)
- Pizza Maker & Kitche N Crew Evaluations: Type of EvaluationDocument3 pagesPizza Maker & Kitche N Crew Evaluations: Type of Evaluationhari juharaNo ratings yet
- 01 eLMS Activity 1 Network TechnologyDocument2 pages01 eLMS Activity 1 Network Technologybasahara sengokuNo ratings yet
- OR Review 3 Ppt.Document36 pagesOR Review 3 Ppt.jay kusainNo ratings yet
- 3GU7 Testimony of Unexpected Situations - Printable Materials - 2022-2023Document35 pages3GU7 Testimony of Unexpected Situations - Printable Materials - 2022-2023Elsa Patricia Parra VázquezNo ratings yet
- Finding Solar Potential Using QGISDocument4 pagesFinding Solar Potential Using QGISJailly AceNo ratings yet
- What Is The Difference Between Cement and Concrete?Document26 pagesWhat Is The Difference Between Cement and Concrete?Al-Buruj InstituteNo ratings yet
- Order Form Dedicated Internet Local: Customer InformationDocument5 pagesOrder Form Dedicated Internet Local: Customer InformationtejoajaaNo ratings yet
- Repair-Training Quotation: Dododo Medical Equipment Service Co.,LtdDocument1 pageRepair-Training Quotation: Dododo Medical Equipment Service Co.,LtdPhong DoNo ratings yet
- Single Crystals, Powders and TwinsDocument48 pagesSingle Crystals, Powders and TwinsJabbar AkbarNo ratings yet
- Sa Pula, Sa PutiDocument84 pagesSa Pula, Sa PutiLuvina Amor Belarma0% (2)
Thermogravimetric Analysis of Calcium Oxalate
Thermogravimetric Analysis of Calcium Oxalate
Uploaded by
akash babelOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermogravimetric Analysis of Calcium Oxalate
Thermogravimetric Analysis of Calcium Oxalate
Uploaded by
akash babelCopyright:
Available Formats
ebatco Exponential Business and Technologies Company
Bridge You and Nano
______________________________________________________________________________
Thermogravimetric Analysis of Calcium Oxalate
Thermogravimetric analysis (TGA) is a type of analysis that determines the mass change of a
sample over time as it is heated. This analysis requires that the test instrument be able to
accurately measure mass, temperature, and temperature change. Typically, samples are analyzed
in an inert atmosphere although an oxidizing or reducing atmosphere can be used when
necessary. TGA is widely employed in research and development, testing and characterization of
all kinds of materials from metals and alloys to polymeric and ceramic composites. Typical
applications of TGA may include determination of polymer degradation and decomposition
temperatures, moisture content of materials, oxidation resistance and dynamics, volatile and non-
volatile components, thermal stability, etc.
Thermal decomposition is the process in which a substance decomposes due to the application of
heat. The phenomenon is common to most organic substances and occurs in many inorganic
substances as well. Certain substances can undergo multiple decomposition reactions, each at a
different temperature. Using thermogravimetry, the decomposition temperature and mass loss of
each reaction can be determined.
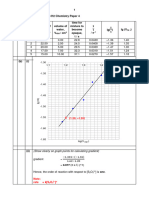
Figure 1. Thermal Decomposition of Calcium Oxalate Monohydrate.
______________________________________________________________________________
Address: 7154 Shady Oak Road, Eden Prairie, MN 55344, U.S.A.
Tel: +1 (952) 334-5486, Fax: +1 (952) 746-8086, Email: info@ebatco.com
www.ebatco.com
ebatco Exponential Business and Technologies Company
Bridge You and Nano
______________________________________________________________________________
Calcium Oxalate Monohydrate, CaC2O4•H2O, is a useful industrial compound used to make
oxalic acid, organic oxalates, and glazes. Thermal decomposition data for CaC2O4•H2O was
obtained using a Simultaneous TG-DTA/DSC Apparatus STA 449 F3 Jupiter (Netszch,
Germany).
Thermal decomposition of CaC2O4•H2O occurs in three distinct steps, as can be seen in Figure 1.
The theoretical mass loss during each step can be calculated using the molar masses of the
individual components. Table 1 shows the decomposition reactions that occur at each step as
well as the theoretical and measured mass loss for each step.
Table 1 Thermal Decomposition Reactions of Calcium Oxalate Monohydrate
Theoretical Mass Measured Mass
Step Reaction % Error
Loss Loss
1 CaC2O4•H2O → CaC2O4 + H2O 12.33% 12.30% -0.24%
2 CaC2O4 → CaCO3 + CO 19.17% 18.90% -1.41%
3 CaCO3 → CaO + CO2 30.12% 29.93% -0.63%
The measured mass losses during steps 1 to 3 closely match the predicted mass losses, which
verify the theoretical predictions of the thermal decomposition for the calcium oxalate
monohydrate. It is interesting to note the slight difference in the mass loss between theoretical
calculation and actual measurement for decomposition in step 2. According to research, this is
most likely due to disproportionation (a type of redox reaction during which a reactant is
simultaneously oxidized and reduced, thus forming two different products) of CO into CO 2 and
carbon. This disproportionation is highly dependent on the impurities within the sample as well
as the cleanliness and material of the sample holder.
______________________________________________________________________________
Address: 7154 Shady Oak Road, Eden Prairie, MN 55344, U.S.A.
Tel: +1 (952) 334-5486, Fax: +1 (952) 746-8086, Email: info@ebatco.com
www.ebatco.com
You might also like
- Chemical Equilibria Tutorial With AnsDocument6 pagesChemical Equilibria Tutorial With AnsDomNo ratings yet
- Free Fall Colla'seDocument3 pagesFree Fall Colla'sesendra20No ratings yet
- HW#3 Optoelectronics 109ADocument2 pagesHW#3 Optoelectronics 109ALuo Mark100% (1)
- Contoh Soal Dan Penyelesain Ke2Document4 pagesContoh Soal Dan Penyelesain Ke2Siti HannaNo ratings yet
- Chemical EquilibriumDocument18 pagesChemical EquilibriumCarbuncle JonesNo ratings yet
- PE - Online SKOLTECH PDFDocument14 pagesPE - Online SKOLTECH PDFOscar100% (1)
- HW 8Document2 pagesHW 8Hai Pham0% (1)
- Emcf 5 F14Document4 pagesEmcf 5 F14ShitPre SuperNo ratings yet
- Sol Ch5 Part2Document5 pagesSol Ch5 Part2mazharNo ratings yet
- Chem 11 Final Exam Review KeyDocument12 pagesChem 11 Final Exam Review Keyboriana72No ratings yet
- Process Dynamics and Control, Ch. 24 Solution ManualDocument27 pagesProcess Dynamics and Control, Ch. 24 Solution ManualBen Spearman100% (1)
- Solution Manual PDFDocument162 pagesSolution Manual PDFBerihu GirmayNo ratings yet
- Final Exam 2012Document12 pagesFinal Exam 2012Mat MorashNo ratings yet
- Earthing Design CalculationsDocument7 pagesEarthing Design CalculationsVamsiNo ratings yet
- ASTM C-40 Tester: Ordering InformationDocument1 pageASTM C-40 Tester: Ordering InformationImber Rene Salgueiro HuancaNo ratings yet
- Engr-2500u Midterm SolutionsDocument6 pagesEngr-2500u Midterm SolutionsAbdullah AlshihriNo ratings yet
- Reactors 2 SolutionsDocument4 pagesReactors 2 Solutionsnripesh_pokhrel100% (2)
- CH 5 PT 1Document3 pagesCH 5 PT 1Jennifer Chang100% (1)
- Solutions Hints 2Document6 pagesSolutions Hints 2iftikhar4498929No ratings yet
- Set6ans 10Document4 pagesSet6ans 10Natália FerreiraNo ratings yet
- Problem Set 3Document3 pagesProblem Set 3AshutoshKumarNo ratings yet
- AIEEE - 2011 Paper With Solutions For Physics, Chemistry and MathsDocument16 pagesAIEEE - 2011 Paper With Solutions For Physics, Chemistry and Mathsstudysteps.inNo ratings yet
- Solutions ManualDocument17 pagesSolutions ManualTzu-li LiuNo ratings yet
- Homework Solutions Lamarsh Chap 5Document10 pagesHomework Solutions Lamarsh Chap 5MarcoMoraNo ratings yet
- 2018 H2 Chemistry Paper 4 (Ans)Document8 pages2018 H2 Chemistry Paper 4 (Ans)Justin GohNo ratings yet
- Code 0 p2 SolutionDocument38 pagesCode 0 p2 Solutionanon020202No ratings yet
- Natural Convection Flow Over Flat Plate Integral SolutionDocument11 pagesNatural Convection Flow Over Flat Plate Integral Solutionsandyengineer13No ratings yet
- Lamarsh ErrataDocument2 pagesLamarsh ErrataMuhammad AbdullahNo ratings yet
- Assignment 1 - Chapter 3 AnswerDocument9 pagesAssignment 1 - Chapter 3 AnswerHarryzam MartelNo ratings yet
- STA 247 - Answers For Practice Problem Set #1Document5 pagesSTA 247 - Answers For Practice Problem Set #1aakasNo ratings yet
- ACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1Document10 pagesACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1ofji4oNo ratings yet
- CHEM1070B - Assignment 3 KeyDocument5 pagesCHEM1070B - Assignment 3 Keymakabigail7No ratings yet
- ch4-6 SlipDocument5 pagesch4-6 SlipAyesha Farooq100% (1)
- Engineering Materials 2 - Chapter 18Document4 pagesEngineering Materials 2 - Chapter 18Andres PerezNo ratings yet
- ENGRD 221 - Thermodynamics (Prof. N. Zabaras) Prelim IDocument9 pagesENGRD 221 - Thermodynamics (Prof. N. Zabaras) Prelim IMurat TülekNo ratings yet
- Modelling Reactors Kinetics PDFDocument65 pagesModelling Reactors Kinetics PDFSachini SarthchandraNo ratings yet
- Chapter 3 OxidationDocument49 pagesChapter 3 OxidationsunNo ratings yet
- Problem Set 1 SolutionsDocument4 pagesProblem Set 1 SolutionsAnshu Kumar GuptaNo ratings yet
- Problem 1. A conducting slab: I ikz−ωtDocument38 pagesProblem 1. A conducting slab: I ikz−ωtHaroonRashidNo ratings yet
- Workbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsDocument14 pagesWorkbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsShubham mishraNo ratings yet
- Problems SetDocument10 pagesProblems SetSajith KurianNo ratings yet
- Corrosion Assignment-2018Document2 pagesCorrosion Assignment-2018Huy An Mai0% (2)
- Peak Position PDFDocument56 pagesPeak Position PDFkumar ujjwalNo ratings yet
- Rate Expression and Activation Energy: 1a. (1 Mark)Document7 pagesRate Expression and Activation Energy: 1a. (1 Mark)senna0% (1)
- CHM3010 Module Thermodynamic-AnsDocument2 pagesCHM3010 Module Thermodynamic-Ansnur hashimahNo ratings yet
- 2006 JC 1 H2 JCT & Promo - Differential EquationsDocument3 pages2006 JC 1 H2 JCT & Promo - Differential EquationsOccamsRazorNo ratings yet
- ch4 ExamplesDocument11 pagesch4 ExamplesSary KilanyNo ratings yet
- ch19 PDFDocument24 pagesch19 PDFRodrigo S QuirinoNo ratings yet
- Tarea 2 FisicaDocument5 pagesTarea 2 FisicaNestor UlloaNo ratings yet
- Worksheet 2Document5 pagesWorksheet 2Theødřøš ÄbNo ratings yet
- 849sample PDFDocument16 pages849sample PDFAryan AsharNo ratings yet
- AJC H2CHEM 2007 Prelims Paper 1Document15 pagesAJC H2CHEM 2007 Prelims Paper 1chuasioklengNo ratings yet
- Resolução Exercs. Cienc. Dos MateriaisDocument19 pagesResolução Exercs. Cienc. Dos MateriaisRafael AraújoNo ratings yet
- Vakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6Document15 pagesVakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6vigiraneza0No ratings yet
- Chapter 7 Diffusion in SolidsDocument39 pagesChapter 7 Diffusion in SolidsNayak Swaraj PankajNo ratings yet
- Transport Processes in The Environment 5Document23 pagesTransport Processes in The Environment 5Amit KumarNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet
- Atomic Absorption Spectroscopy: International Atomic Absorption Spectroscopy ConferenceFrom EverandAtomic Absorption Spectroscopy: International Atomic Absorption Spectroscopy ConferenceR. M. DagnallNo ratings yet
- Corrosion Inhibition Performance of Copper Carbonate in MEADocument5 pagesCorrosion Inhibition Performance of Copper Carbonate in MEAdow2008No ratings yet
- Physical/ Chemical Changes: CGA #2 ReviewDocument8 pagesPhysical/ Chemical Changes: CGA #2 Reviewengchemistry18No ratings yet
- Eugenio Ochoa GonzalezDocument3 pagesEugenio Ochoa GonzalezEugenio Ochoa GonzalezNo ratings yet
- GloverDocument272 pagesGlovermidialaoropesaNo ratings yet
- MB0052-Strategic Management and Business Policy (Assignment-1)Document9 pagesMB0052-Strategic Management and Business Policy (Assignment-1)Anil KumarNo ratings yet
- Villa As ParadigmDocument4 pagesVilla As ParadigmPaul Turner100% (1)
- Push Button Typical WiringDocument12 pagesPush Button Typical Wiringstrob1974No ratings yet
- Indirani College of Nursing: Level of Student - B.SC (N) Ii Yrs TractionDocument7 pagesIndirani College of Nursing: Level of Student - B.SC (N) Ii Yrs TractiondhanasundariNo ratings yet
- MR - Tushar Thombare Resume PDFDocument3 pagesMR - Tushar Thombare Resume PDFprasadNo ratings yet
- Jak Na Power Bi Cheat SheetDocument3 pagesJak Na Power Bi Cheat SheetenggdkteNo ratings yet
- Trade WorksheetDocument11 pagesTrade WorksheetAsma KamranNo ratings yet
- Unit 1 - 18EC61Document93 pagesUnit 1 - 18EC61Pritam SarkarNo ratings yet
- Mathematics Grade 10Document270 pagesMathematics Grade 10TammanurRaviNo ratings yet
- Tugas Uas Miss LennyDocument8 pagesTugas Uas Miss LennyZiyan Khoirun hakimNo ratings yet
- Genesis g16Document2 pagesGenesis g16Krist UtamaNo ratings yet
- Standard Electrode Potentials & CellsDocument3 pagesStandard Electrode Potentials & Cellsmy nameNo ratings yet
- Control System Kec 602Document2 pagesControl System Kec 602Nitya MishraNo ratings yet
- 128M (8Mx16) GDDR SDRAM: HY5DU281622ETDocument34 pages128M (8Mx16) GDDR SDRAM: HY5DU281622ETBoris LazarchukNo ratings yet
- Adobe Photoshop CS6 Exam SimulationDocument6 pagesAdobe Photoshop CS6 Exam SimulationMuhammad NadhirNo ratings yet
- EV-100ZX Motor Controller Description & RepairDocument95 pagesEV-100ZX Motor Controller Description & RepairMario Aguirre100% (1)
- Pizza Maker & Kitche N Crew Evaluations: Type of EvaluationDocument3 pagesPizza Maker & Kitche N Crew Evaluations: Type of Evaluationhari juharaNo ratings yet
- 01 eLMS Activity 1 Network TechnologyDocument2 pages01 eLMS Activity 1 Network Technologybasahara sengokuNo ratings yet
- OR Review 3 Ppt.Document36 pagesOR Review 3 Ppt.jay kusainNo ratings yet
- 3GU7 Testimony of Unexpected Situations - Printable Materials - 2022-2023Document35 pages3GU7 Testimony of Unexpected Situations - Printable Materials - 2022-2023Elsa Patricia Parra VázquezNo ratings yet
- Finding Solar Potential Using QGISDocument4 pagesFinding Solar Potential Using QGISJailly AceNo ratings yet
- What Is The Difference Between Cement and Concrete?Document26 pagesWhat Is The Difference Between Cement and Concrete?Al-Buruj InstituteNo ratings yet
- Order Form Dedicated Internet Local: Customer InformationDocument5 pagesOrder Form Dedicated Internet Local: Customer InformationtejoajaaNo ratings yet
- Repair-Training Quotation: Dododo Medical Equipment Service Co.,LtdDocument1 pageRepair-Training Quotation: Dododo Medical Equipment Service Co.,LtdPhong DoNo ratings yet
- Single Crystals, Powders and TwinsDocument48 pagesSingle Crystals, Powders and TwinsJabbar AkbarNo ratings yet
- Sa Pula, Sa PutiDocument84 pagesSa Pula, Sa PutiLuvina Amor Belarma0% (2)