Professional Documents
Culture Documents
215-216 HH W13-Nmr-Iii PDF
215-216 HH W13-Nmr-Iii PDF
Uploaded by
Rodrigo GarciaCopyright:
Available Formats
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 13C NMRDocument28 pages13C NMRArjun MaharajNo ratings yet
- NMR 13C 13 QuesDocument52 pagesNMR 13C 13 QuesgussalimNo ratings yet
- Notes 14C CNMRDocument5 pagesNotes 14C CNMRTux BsdNo ratings yet
- M.SC NMR SpectrosDocument72 pagesM.SC NMR SpectrosShweta BishtNo ratings yet
- NMR SpectrosDocument32 pagesNMR SpectrosIndhuja Preethi BNo ratings yet
- C-13 NMRDocument27 pagesC-13 NMRDeby Aulia Tri NNo ratings yet
- 13C NMR Spectroscopy Power Point PresentationDocument34 pages13C NMR Spectroscopy Power Point PresentationOti DeeaNo ratings yet
- 13c NMR SpectrosDocument29 pages13c NMR SpectrosKudzai ChidzodzoNo ratings yet
- Introduction To NMR Spectroscopy: Part II (C-NMR)Document21 pagesIntroduction To NMR Spectroscopy: Part II (C-NMR)andi evi febriantiNo ratings yet
- Course Notes NMR (Day 4)Document12 pagesCourse Notes NMR (Day 4)hmhdecNo ratings yet
- Solve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation PatternDocument8 pagesSolve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation Patternramesh pokhrelNo ratings yet
- Lecture7_CH317_2020_Fall_NMR_part3 modified answersDocument13 pagesLecture7_CH317_2020_Fall_NMR_part3 modified answersshanicejames7867No ratings yet
- L3 NMR 1Document13 pagesL3 NMR 1Cheng FuNo ratings yet
- 3 - Carbon-13-NMRDocument21 pages3 - Carbon-13-NMRahmed mohamedNo ratings yet
- Alkyne-Anna IN CLASS 1Document26 pagesAlkyne-Anna IN CLASS 1Siti Farhanah Mohd NasirNo ratings yet
- 39 SpectrosDocument9 pages39 SpectrosDinel JosephNo ratings yet
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- 13C NMRDocument40 pages13C NMRKrishna BurakaNo ratings yet
- 13 C NMR InterpretationDocument9 pages13 C NMR InterpretationDavid ScoNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument39 pagesNuclear Magnetic Resonance (NMR) SpectrosknkoradiyaNo ratings yet
- 13CNMRfor322 PDFDocument45 pages13CNMRfor322 PDFshaniNo ratings yet
- Alkena Dan Aromatis NMRDocument41 pagesAlkena Dan Aromatis NMRlucy7696No ratings yet
- Stereochemistry Assignment #2 2018-2019 ANSWERSDocument3 pagesStereochemistry Assignment #2 2018-2019 ANSWERSZoe NorvilleNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument24 pagesNuclear Magnetic Resonance (NMR) Spectroshatna sayNo ratings yet
- Ef0c00890 Si 001Document6 pagesEf0c00890 Si 001Austin SmithNo ratings yet
- SPEC6A Problems 3-5 AnswersDocument19 pagesSPEC6A Problems 3-5 Answersnonkululekomoya26No ratings yet
- Alkuna: Ichtheothereol (Racun Panah Amazonian)Document15 pagesAlkuna: Ichtheothereol (Racun Panah Amazonian)Anita puspitasariNo ratings yet
- Chap. 5 Reactive Intermediates: Energy SurfaceDocument20 pagesChap. 5 Reactive Intermediates: Energy SurfaceAnil KumarNo ratings yet
- NMR 27 APRIL To 27 May 20Document34 pagesNMR 27 APRIL To 27 May 20Quratul AinNo ratings yet
- NMR introductionDocument3 pagesNMR introductionntokozocecilia81No ratings yet
- Twelve Easy Problems: Combined Problems Formula + Infrared + NMRDocument32 pagesTwelve Easy Problems: Combined Problems Formula + Infrared + NMRRona Prima LarasatiNo ratings yet
- ws4 PDFDocument5 pagesws4 PDFmohammedabubakrNo ratings yet
- Approximate 1H and 13C NMR ShiftsDocument5 pagesApproximate 1H and 13C NMR ShiftsCamilo AndresNo ratings yet
- Spectroscopy Nuclear Magnetic ResonanceDocument54 pagesSpectroscopy Nuclear Magnetic ResonanceDamar Nurwahyu BimaNo ratings yet
- CHE320 OS Test 1 Key QN 4Document2 pagesCHE320 OS Test 1 Key QN 4PdomNo ratings yet
- H/NMR MetodaDocument31 pagesH/NMR MetodaAlexandra MilenkovicNo ratings yet
- Stereochemistry of SN Reactions PPT - Copy - Copy-1Document28 pagesStereochemistry of SN Reactions PPT - Copy - Copy-1Vidya Rani100% (2)
- Nucleophilic Substitution ReactionDocument17 pagesNucleophilic Substitution ReactionRojo JohnNo ratings yet
- Réarrangement RadicalaireDocument5 pagesRéarrangement RadicalaireRaf Belo100% (1)
- Chapter 10 - Amides - Sparkman2011Document6 pagesChapter 10 - Amides - Sparkman2011elenitabastosNo ratings yet
- Introductory Organic ChemistryDocument43 pagesIntroductory Organic ChemistryCharlotte TalyorNo ratings yet
- 13-C NMR-09Document27 pages13-C NMR-09M Nur M. MahmudNo ratings yet
- Chapter 13.2 - RothDocument37 pagesChapter 13.2 - Rothkishan patelNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Chapter 13: Nuclear Magnetic Resonance (NMR) SpectrosDocument20 pagesChapter 13: Nuclear Magnetic Resonance (NMR) SpectrosWwe 2No ratings yet
- Alkanes - Arenes-1Document46 pagesAlkanes - Arenes-1deyar.deyar123No ratings yet
- Organic Chem. NotesDocument80 pagesOrganic Chem. NoteselcarlsansNo ratings yet
- Carboxylic Acid & Derivatives-02 - Solved ProblemsDocument15 pagesCarboxylic Acid & Derivatives-02 - Solved ProblemsRaju SinghNo ratings yet
- SCHB032 NMR Part2redoDocument29 pagesSCHB032 NMR Part2redoemjayNo ratings yet
- Stereochirality R or SDocument52 pagesStereochirality R or SnifafaniNo ratings yet
- Haloalkanes and HaloarenesDocument23 pagesHaloalkanes and Haloarenesn29334456No ratings yet
- Spec Fragment Example Solution F14Document5 pagesSpec Fragment Example Solution F14admiraldamian67No ratings yet
- S DQPK SNyf 9 WNJHups Y4 UDocument38 pagesS DQPK SNyf 9 WNJHups Y4 UKushal DubeyNo ratings yet
- NMR Hly09Document44 pagesNMR Hly09Giang NguyenNo ratings yet
- C 13 NMRDocument75 pagesC 13 NMRalwysyahNo ratings yet
- Lecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFDocument8 pagesLecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFPhillip WachowiakNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Future Tense Exercises Fun Activities Games Grammar Drills 1032Document1 pageFuture Tense Exercises Fun Activities Games Grammar Drills 1032Rodrigo GarciaNo ratings yet
- Recent Advances in The Catalytic Asymmetric Synthesis of B-Amino AcidsDocument36 pagesRecent Advances in The Catalytic Asymmetric Synthesis of B-Amino AcidsRodrigo GarciaNo ratings yet
- Future Tense Exercises Fun Activities Games Grammar Drills 1032Document1 pageFuture Tense Exercises Fun Activities Games Grammar Drills 1032Rodrigo GarciaNo ratings yet
- Maaz International: Subject: Quotation For Relumins 15000mg Advance Glutathione - OralDocument2 pagesMaaz International: Subject: Quotation For Relumins 15000mg Advance Glutathione - OralRodrigo GarciaNo ratings yet
- Unit 9. Reported Speech: Y MiscellaneaDocument11 pagesUnit 9. Reported Speech: Y MiscellaneaRodrigo GarciaNo ratings yet
- ' - Ococh: Bacterio,':,ta'i'r?Document5 pages' - Ococh: Bacterio,':,ta'i'r?Rodrigo GarciaNo ratings yet
- A System.: (Received in UK 2 December 1986)Document6 pagesA System.: (Received in UK 2 December 1986)Rodrigo GarciaNo ratings yet
- Solvent-Free Reductive Amination: An Organic Chemistry ExperimentDocument3 pagesSolvent-Free Reductive Amination: An Organic Chemistry ExperimentRodrigo GarciaNo ratings yet
- Cho2005 PDFDocument10 pagesCho2005 PDFRodrigo GarciaNo ratings yet
- Organic ReactionsDocument37 pagesOrganic ReactionsRodrigo GarciaNo ratings yet
- AP Lab Manual 18 - Kinetics of A ReactionDocument13 pagesAP Lab Manual 18 - Kinetics of A ReactionRodrigo GarciaNo ratings yet
- Lecture 8 XPSDocument62 pagesLecture 8 XPSarulmuruguNo ratings yet
- Chemistry Module 2 Part 3Document60 pagesChemistry Module 2 Part 3RiyazNo ratings yet
- HybridizationDocument50 pagesHybridizationmar_ouq63% (8)
- Basics of Organic Chemistry B Paaras Thakur @livedailyjeeDocument132 pagesBasics of Organic Chemistry B Paaras Thakur @livedailyjeeEluri YadaiahNo ratings yet
- Module-3: Recombination in SemiconductorsDocument13 pagesModule-3: Recombination in SemiconductorsKARUTURI AKASH 17BEC0396No ratings yet
- CHEM 221/PHY 335 - Molecular Symmetry IDocument34 pagesCHEM 221/PHY 335 - Molecular Symmetry Ipaul javed0% (1)
- Polarity of MoleculesDocument85 pagesPolarity of MoleculesAislinn Sheen AcasioNo ratings yet
- Polymer Impregnated Turquoise PDFDocument3 pagesPolymer Impregnated Turquoise PDF8duke8No ratings yet
- Molecular Probes Handbook - A Guide To Fluorescent Probes and Labeling Technologies 11th Edition (2010)Document973 pagesMolecular Probes Handbook - A Guide To Fluorescent Probes and Labeling Technologies 11th Edition (2010)Lei FanNo ratings yet
- 11 Chemistry Solved Questions Chapter 4Document4 pages11 Chemistry Solved Questions Chapter 4amal gainNo ratings yet
- CHM260 Basic Instrumental AnalysisDocument6 pagesCHM260 Basic Instrumental Analysisfatin harris100% (1)
- IR Spectroscopy 4Document8 pagesIR Spectroscopy 4alina.tlekkabylova270202No ratings yet
- Heteroatomic Molecular Orbitals: Fill Mos in OrderDocument2 pagesHeteroatomic Molecular Orbitals: Fill Mos in OrdersernaNo ratings yet
- Chemical Bonding and Molecular StructureDocument6 pagesChemical Bonding and Molecular StructureSahil BhardwajNo ratings yet
- Advanced Inorganic Chemistry - ROBERT L. CARTERDocument15 pagesAdvanced Inorganic Chemistry - ROBERT L. CARTERBRUNO RAMOS DE LIMANo ratings yet
- Chemistry A Molecular Approach With Masteringchemistry 2nd Edition Tro Test BankDocument38 pagesChemistry A Molecular Approach With Masteringchemistry 2nd Edition Tro Test Bankfoliosemyophanr2cl100% (19)
- Spectrophotometry FundamentalsDocument15 pagesSpectrophotometry FundamentalschipulinoNo ratings yet
- Biochemistry BasicsDocument22 pagesBiochemistry Basicsjazlamba09No ratings yet
- Chem 222 Sample Exam 3Document8 pagesChem 222 Sample Exam 3Yarys YauNo ratings yet
- Aes NotesDocument13 pagesAes Notesp.ishaanpawarNo ratings yet
- M3 Intermolecular Forces of AttractionDocument17 pagesM3 Intermolecular Forces of AttractionEvangeline AgtarapNo ratings yet
- DFT and TD-DFT Studies On Copper (II) Complexes With Tripodal Tetramine LigandsDocument6 pagesDFT and TD-DFT Studies On Copper (II) Complexes With Tripodal Tetramine LigandsAravind KNo ratings yet
- Synchrotron - Beamline - Capabilities 2Document1 pageSynchrotron - Beamline - Capabilities 2Erma NirmalaNo ratings yet
- Report 1 - Exper 4Document4 pagesReport 1 - Exper 4Walmiria LimaNo ratings yet
- 2019 - 2020 Chapter 2 Atomic Structure SK015Document71 pages2019 - 2020 Chapter 2 Atomic Structure SK015Jia En TanNo ratings yet
- AP Inter 1st Year Chemistry Important Questions Chapter 1 Atomic Structure - AP Board SolutionsDocument8 pagesAP Inter 1st Year Chemistry Important Questions Chapter 1 Atomic Structure - AP Board SolutionsN JASWANTHNo ratings yet
- Admatel Opens For Business Final - March 27 2013Document3 pagesAdmatel Opens For Business Final - March 27 2013EliasA.TiongkiaoNo ratings yet
- Molecular Chemical Bonding NotesDocument2 pagesMolecular Chemical Bonding NotesMeera KumarNo ratings yet
- Delhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyDocument6 pagesDelhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyShashwatNo ratings yet
- ENVE 450 - Lecture 5 - AAS PDFDocument6 pagesENVE 450 - Lecture 5 - AAS PDFclaudiutp100% (1)
215-216 HH W13-Nmr-Iii PDF
215-216 HH W13-Nmr-Iii PDF
Uploaded by
Rodrigo GarciaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
215-216 HH W13-Nmr-Iii PDF
215-216 HH W13-Nmr-Iii PDF
Uploaded by
Rodrigo GarciaCopyright:
Available Formats
215-216 HH W13 Notes - Dr.
Masato Koreeda Date: January 9, 2013
Topic: _NMR-III (C-13 NMR)_ page 1 of 2.
13
C NMR Spectroscopy
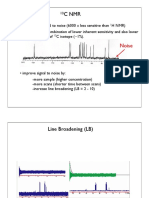
(1) 13C Isotope (I = 1/2; the same as 1H)
Natural abundance of 13C is only 1% [99% of carbon is 12C (I = 0)].
For example, for CH3CH2OH: A probability of finding 13C in the CH3 is 0.01 (1%) and
a probability of finding 13C in the CH2 is 0.01 (1%).
Thus, a probability of finding 13C in both the CH3 and CH2 at the same time is 0.001 (1% of 1%).
Therefore, the contribution of 13C-13C coupling in natural abundance 13C NMR may be ignored.
However, 13C found at any 1H-attached carbon exhibits spin-spin coupling with the 1H(s) (one-bond
coupling; 1J13C,1H). In order to avoid complications due to these 13C-1H couplings, 13C NMR spectra are
usually obtained by eliminating these couplings by irradiating the entire 1H NMR absorption range
(called decoupling). Consequently, all 13C NMR peaks are observed as singlets. This mode of
obtaining 13C NMR spectra is called a proton-decoupled (or broad-band decoupled) method.
(2) Proton-decoupled 13C NMR Spectra
As in the case of 1H NMR, 13C NMR spectra are obtained typically in CDCl3 with tetramethylsilane
(TMS) as internal reference, i.e., δ = 0 ppm for TMS methyl carbons. Most of the 13C NMR chemical
shift values fall in the range between 0 and 220 ppm. For representative 13C NMR chemical shifts, see
Table 11.2, p. 403 of Ege’s book.
aromatic C's
O D: I = 1
O on the basis of 2 I + 1,
H3C H becomes a triplet 1
i.e., J13C-D
6-chemically different C groups -O-CH3
CDCl3 TMS
H
C
O
191 165 114 78 56 0 ppm (δ)
132 130
(a) Unlike 1H NMR, the intensity of each of the 13C peaks is not proportional to the number of 13C
nuclei belonging to each peak.
(b) 13C NMR chemical shifts are predictable primarily based upon the electron density on each C.
H CH3
e.g., -CH2-CH3 10-15 ppm -O-CH2-CH3 | |
-O-C-CH3 -O-C-CH3
60-65 ppm | |
-O-CH3 55 ppm 75-80 ppm CH 90-100 ppm CH
3 3
(c) The chemical shifts of alkynic sp carbons are unusual as in the case of alkynic Hs in 1H NMR.
However, allenic sp carbons have expected chemical shifts (~ 200 ppm).
sp2
67-70 ppm sp cf.
H3C
R-C C-H C=C=CH2 72 ppm CH3
H3C CH3 143.7 ppm
H3C
74-85 ppm C C
93.4 ppm 207 ppm H3C C CH2
H2
R-C N 114.4 ppm
These are quite high-field shifted
117-125 ppm for sp2 carbons!
215-216 HH W13 Notes - Dr. Masato Koreeda Date: January 9, 2013
Topic: _NMR-III (C-13 NMR)_ page 2 of 2.
(d) Unlike 1H NMR, alkenic and aromatic carbons have similar chemical shifts (δ 110 – 140 ppm)
(i.e., no significant ring current effect observed in C-13 NMR).
(e) Chemical shifts of aromatic carbons: predictable on the basis of the resonance and inductive
effects of the substituents on the ring.
155.4 ppm! - due to the inductive
effect of the oxygen atom.
OH O O O O
H H H H
115.8 ppm
129.9 ppm increased p-electron
up-field shifted density due to the resonance
121.2 ppm from 128.5 ppm
effects caused by the OH.
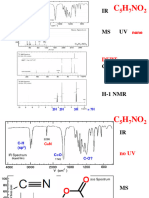
(3) Proton-coupled 13C NMR Spectra
By irradiating slightly off the 1H NMR region, each of the 13C NMR peaks can be designed to exhibit
couplings with the 1H nuclei attached to the C, i.e., one-bond 13C-1H coupling(s).
Under such conditions: a CH3 (methyl) carbon appears as a quartet.
a CH2 (methylene) carbon appears as a triplet.
a CH (methine) carbon appears as a doublet.
a quaternary carbon appears as a singlet.
O aromatic C's Proton-decoupled
C-13 spectrum D: I = 1
O on the basis of 2 I + 1,
H3C H in CDCl3
becomes a triplet 1
i.e., J13C-D
6-chemically different C groups -O-CH3
CDCl3 TMS
H
C
O
191 165 114 78 56 0 ppm (δ)
132 130
Proton-coupled
C-13 spectrum
doublet in CDCl3
doublet
quartet CH3
doublet CH3 quartet
singlet singlet CDCl3 TMS [=Si(CH3)4]
H °
C °
O
191 165 132 114 H 78 56 0 ppm (δ)
130
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 13C NMRDocument28 pages13C NMRArjun MaharajNo ratings yet
- NMR 13C 13 QuesDocument52 pagesNMR 13C 13 QuesgussalimNo ratings yet
- Notes 14C CNMRDocument5 pagesNotes 14C CNMRTux BsdNo ratings yet
- M.SC NMR SpectrosDocument72 pagesM.SC NMR SpectrosShweta BishtNo ratings yet
- NMR SpectrosDocument32 pagesNMR SpectrosIndhuja Preethi BNo ratings yet
- C-13 NMRDocument27 pagesC-13 NMRDeby Aulia Tri NNo ratings yet
- 13C NMR Spectroscopy Power Point PresentationDocument34 pages13C NMR Spectroscopy Power Point PresentationOti DeeaNo ratings yet
- 13c NMR SpectrosDocument29 pages13c NMR SpectrosKudzai ChidzodzoNo ratings yet
- Introduction To NMR Spectroscopy: Part II (C-NMR)Document21 pagesIntroduction To NMR Spectroscopy: Part II (C-NMR)andi evi febriantiNo ratings yet
- Course Notes NMR (Day 4)Document12 pagesCourse Notes NMR (Day 4)hmhdecNo ratings yet
- Solve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation PatternDocument8 pagesSolve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation Patternramesh pokhrelNo ratings yet
- Lecture7_CH317_2020_Fall_NMR_part3 modified answersDocument13 pagesLecture7_CH317_2020_Fall_NMR_part3 modified answersshanicejames7867No ratings yet
- L3 NMR 1Document13 pagesL3 NMR 1Cheng FuNo ratings yet
- 3 - Carbon-13-NMRDocument21 pages3 - Carbon-13-NMRahmed mohamedNo ratings yet
- Alkyne-Anna IN CLASS 1Document26 pagesAlkyne-Anna IN CLASS 1Siti Farhanah Mohd NasirNo ratings yet
- 39 SpectrosDocument9 pages39 SpectrosDinel JosephNo ratings yet
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- 13C NMRDocument40 pages13C NMRKrishna BurakaNo ratings yet
- 13 C NMR InterpretationDocument9 pages13 C NMR InterpretationDavid ScoNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument39 pagesNuclear Magnetic Resonance (NMR) SpectrosknkoradiyaNo ratings yet
- 13CNMRfor322 PDFDocument45 pages13CNMRfor322 PDFshaniNo ratings yet
- Alkena Dan Aromatis NMRDocument41 pagesAlkena Dan Aromatis NMRlucy7696No ratings yet
- Stereochemistry Assignment #2 2018-2019 ANSWERSDocument3 pagesStereochemistry Assignment #2 2018-2019 ANSWERSZoe NorvilleNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument24 pagesNuclear Magnetic Resonance (NMR) Spectroshatna sayNo ratings yet
- Ef0c00890 Si 001Document6 pagesEf0c00890 Si 001Austin SmithNo ratings yet
- SPEC6A Problems 3-5 AnswersDocument19 pagesSPEC6A Problems 3-5 Answersnonkululekomoya26No ratings yet
- Alkuna: Ichtheothereol (Racun Panah Amazonian)Document15 pagesAlkuna: Ichtheothereol (Racun Panah Amazonian)Anita puspitasariNo ratings yet
- Chap. 5 Reactive Intermediates: Energy SurfaceDocument20 pagesChap. 5 Reactive Intermediates: Energy SurfaceAnil KumarNo ratings yet
- NMR 27 APRIL To 27 May 20Document34 pagesNMR 27 APRIL To 27 May 20Quratul AinNo ratings yet
- NMR introductionDocument3 pagesNMR introductionntokozocecilia81No ratings yet
- Twelve Easy Problems: Combined Problems Formula + Infrared + NMRDocument32 pagesTwelve Easy Problems: Combined Problems Formula + Infrared + NMRRona Prima LarasatiNo ratings yet
- ws4 PDFDocument5 pagesws4 PDFmohammedabubakrNo ratings yet
- Approximate 1H and 13C NMR ShiftsDocument5 pagesApproximate 1H and 13C NMR ShiftsCamilo AndresNo ratings yet
- Spectroscopy Nuclear Magnetic ResonanceDocument54 pagesSpectroscopy Nuclear Magnetic ResonanceDamar Nurwahyu BimaNo ratings yet
- CHE320 OS Test 1 Key QN 4Document2 pagesCHE320 OS Test 1 Key QN 4PdomNo ratings yet
- H/NMR MetodaDocument31 pagesH/NMR MetodaAlexandra MilenkovicNo ratings yet
- Stereochemistry of SN Reactions PPT - Copy - Copy-1Document28 pagesStereochemistry of SN Reactions PPT - Copy - Copy-1Vidya Rani100% (2)
- Nucleophilic Substitution ReactionDocument17 pagesNucleophilic Substitution ReactionRojo JohnNo ratings yet
- Réarrangement RadicalaireDocument5 pagesRéarrangement RadicalaireRaf Belo100% (1)
- Chapter 10 - Amides - Sparkman2011Document6 pagesChapter 10 - Amides - Sparkman2011elenitabastosNo ratings yet
- Introductory Organic ChemistryDocument43 pagesIntroductory Organic ChemistryCharlotte TalyorNo ratings yet
- 13-C NMR-09Document27 pages13-C NMR-09M Nur M. MahmudNo ratings yet
- Chapter 13.2 - RothDocument37 pagesChapter 13.2 - Rothkishan patelNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Chapter 13: Nuclear Magnetic Resonance (NMR) SpectrosDocument20 pagesChapter 13: Nuclear Magnetic Resonance (NMR) SpectrosWwe 2No ratings yet
- Alkanes - Arenes-1Document46 pagesAlkanes - Arenes-1deyar.deyar123No ratings yet
- Organic Chem. NotesDocument80 pagesOrganic Chem. NoteselcarlsansNo ratings yet
- Carboxylic Acid & Derivatives-02 - Solved ProblemsDocument15 pagesCarboxylic Acid & Derivatives-02 - Solved ProblemsRaju SinghNo ratings yet
- SCHB032 NMR Part2redoDocument29 pagesSCHB032 NMR Part2redoemjayNo ratings yet
- Stereochirality R or SDocument52 pagesStereochirality R or SnifafaniNo ratings yet
- Haloalkanes and HaloarenesDocument23 pagesHaloalkanes and Haloarenesn29334456No ratings yet
- Spec Fragment Example Solution F14Document5 pagesSpec Fragment Example Solution F14admiraldamian67No ratings yet
- S DQPK SNyf 9 WNJHups Y4 UDocument38 pagesS DQPK SNyf 9 WNJHups Y4 UKushal DubeyNo ratings yet
- NMR Hly09Document44 pagesNMR Hly09Giang NguyenNo ratings yet
- C 13 NMRDocument75 pagesC 13 NMRalwysyahNo ratings yet
- Lecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFDocument8 pagesLecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFPhillip WachowiakNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Future Tense Exercises Fun Activities Games Grammar Drills 1032Document1 pageFuture Tense Exercises Fun Activities Games Grammar Drills 1032Rodrigo GarciaNo ratings yet
- Recent Advances in The Catalytic Asymmetric Synthesis of B-Amino AcidsDocument36 pagesRecent Advances in The Catalytic Asymmetric Synthesis of B-Amino AcidsRodrigo GarciaNo ratings yet
- Future Tense Exercises Fun Activities Games Grammar Drills 1032Document1 pageFuture Tense Exercises Fun Activities Games Grammar Drills 1032Rodrigo GarciaNo ratings yet
- Maaz International: Subject: Quotation For Relumins 15000mg Advance Glutathione - OralDocument2 pagesMaaz International: Subject: Quotation For Relumins 15000mg Advance Glutathione - OralRodrigo GarciaNo ratings yet
- Unit 9. Reported Speech: Y MiscellaneaDocument11 pagesUnit 9. Reported Speech: Y MiscellaneaRodrigo GarciaNo ratings yet
- ' - Ococh: Bacterio,':,ta'i'r?Document5 pages' - Ococh: Bacterio,':,ta'i'r?Rodrigo GarciaNo ratings yet
- A System.: (Received in UK 2 December 1986)Document6 pagesA System.: (Received in UK 2 December 1986)Rodrigo GarciaNo ratings yet
- Solvent-Free Reductive Amination: An Organic Chemistry ExperimentDocument3 pagesSolvent-Free Reductive Amination: An Organic Chemistry ExperimentRodrigo GarciaNo ratings yet
- Cho2005 PDFDocument10 pagesCho2005 PDFRodrigo GarciaNo ratings yet
- Organic ReactionsDocument37 pagesOrganic ReactionsRodrigo GarciaNo ratings yet
- AP Lab Manual 18 - Kinetics of A ReactionDocument13 pagesAP Lab Manual 18 - Kinetics of A ReactionRodrigo GarciaNo ratings yet
- Lecture 8 XPSDocument62 pagesLecture 8 XPSarulmuruguNo ratings yet
- Chemistry Module 2 Part 3Document60 pagesChemistry Module 2 Part 3RiyazNo ratings yet
- HybridizationDocument50 pagesHybridizationmar_ouq63% (8)
- Basics of Organic Chemistry B Paaras Thakur @livedailyjeeDocument132 pagesBasics of Organic Chemistry B Paaras Thakur @livedailyjeeEluri YadaiahNo ratings yet
- Module-3: Recombination in SemiconductorsDocument13 pagesModule-3: Recombination in SemiconductorsKARUTURI AKASH 17BEC0396No ratings yet
- CHEM 221/PHY 335 - Molecular Symmetry IDocument34 pagesCHEM 221/PHY 335 - Molecular Symmetry Ipaul javed0% (1)
- Polarity of MoleculesDocument85 pagesPolarity of MoleculesAislinn Sheen AcasioNo ratings yet
- Polymer Impregnated Turquoise PDFDocument3 pagesPolymer Impregnated Turquoise PDF8duke8No ratings yet
- Molecular Probes Handbook - A Guide To Fluorescent Probes and Labeling Technologies 11th Edition (2010)Document973 pagesMolecular Probes Handbook - A Guide To Fluorescent Probes and Labeling Technologies 11th Edition (2010)Lei FanNo ratings yet
- 11 Chemistry Solved Questions Chapter 4Document4 pages11 Chemistry Solved Questions Chapter 4amal gainNo ratings yet
- CHM260 Basic Instrumental AnalysisDocument6 pagesCHM260 Basic Instrumental Analysisfatin harris100% (1)
- IR Spectroscopy 4Document8 pagesIR Spectroscopy 4alina.tlekkabylova270202No ratings yet
- Heteroatomic Molecular Orbitals: Fill Mos in OrderDocument2 pagesHeteroatomic Molecular Orbitals: Fill Mos in OrdersernaNo ratings yet
- Chemical Bonding and Molecular StructureDocument6 pagesChemical Bonding and Molecular StructureSahil BhardwajNo ratings yet
- Advanced Inorganic Chemistry - ROBERT L. CARTERDocument15 pagesAdvanced Inorganic Chemistry - ROBERT L. CARTERBRUNO RAMOS DE LIMANo ratings yet
- Chemistry A Molecular Approach With Masteringchemistry 2nd Edition Tro Test BankDocument38 pagesChemistry A Molecular Approach With Masteringchemistry 2nd Edition Tro Test Bankfoliosemyophanr2cl100% (19)
- Spectrophotometry FundamentalsDocument15 pagesSpectrophotometry FundamentalschipulinoNo ratings yet
- Biochemistry BasicsDocument22 pagesBiochemistry Basicsjazlamba09No ratings yet
- Chem 222 Sample Exam 3Document8 pagesChem 222 Sample Exam 3Yarys YauNo ratings yet
- Aes NotesDocument13 pagesAes Notesp.ishaanpawarNo ratings yet
- M3 Intermolecular Forces of AttractionDocument17 pagesM3 Intermolecular Forces of AttractionEvangeline AgtarapNo ratings yet
- DFT and TD-DFT Studies On Copper (II) Complexes With Tripodal Tetramine LigandsDocument6 pagesDFT and TD-DFT Studies On Copper (II) Complexes With Tripodal Tetramine LigandsAravind KNo ratings yet
- Synchrotron - Beamline - Capabilities 2Document1 pageSynchrotron - Beamline - Capabilities 2Erma NirmalaNo ratings yet
- Report 1 - Exper 4Document4 pagesReport 1 - Exper 4Walmiria LimaNo ratings yet
- 2019 - 2020 Chapter 2 Atomic Structure SK015Document71 pages2019 - 2020 Chapter 2 Atomic Structure SK015Jia En TanNo ratings yet
- AP Inter 1st Year Chemistry Important Questions Chapter 1 Atomic Structure - AP Board SolutionsDocument8 pagesAP Inter 1st Year Chemistry Important Questions Chapter 1 Atomic Structure - AP Board SolutionsN JASWANTHNo ratings yet
- Admatel Opens For Business Final - March 27 2013Document3 pagesAdmatel Opens For Business Final - March 27 2013EliasA.TiongkiaoNo ratings yet
- Molecular Chemical Bonding NotesDocument2 pagesMolecular Chemical Bonding NotesMeera KumarNo ratings yet
- Delhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyDocument6 pagesDelhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyShashwatNo ratings yet
- ENVE 450 - Lecture 5 - AAS PDFDocument6 pagesENVE 450 - Lecture 5 - AAS PDFclaudiutp100% (1)