Professional Documents
Culture Documents
Assignment L01-T6
Assignment L01-T6
Uploaded by
MawareOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Assignment L01-T6
Assignment L01-T6
Uploaded by
MawareCopyright:
Available Formats
SEPTEMBER 2014 CONFIDENTIAL
ASSIGNMENT (5%)
SECTION : L01-T6
Answer ALL questions
Question 1
(a) An equation to express the Boltzmann’s constant is given as follows.
Nb −V ×g×h
ln =
Na (υ 2 −υ1 )×k×T
Where: Nb and Na = Number of particles, V = Volume, g = Gravitational acceleration,
h = Distance, ν2 and ν1 = Specific volume, T = Absolute temperature, and
k = Boltzmann’s constant (unit J/K).
Determine whether the equation is dimensionally homogenous.
(10 marks)
(b) A mixture with average molecular weight of 51.2 contains 30.0 wt% acetone ,
acetaldehyde, and acetic acid. It is found that the volume of acetone as 150.0 ft 3.
i. Solve the mass fractions for acetaldehyde and acetic acid in the mixture.
(4 marks)
ii. Determine the number of moles (in lbmol) of each compound in the mixture.
(7 marks)
3
iii. Solve the volume of acetaldehyde and acetic acid (in ft ).
(4 marks)
Question 2
(a) The density of a fluid can be represented by the equation below
−5
ρ=45.0exp(5.77×10 P)
Where; ρ is the density (in kg/m3) and P is the pressure (in N/m2).
i. Identify the units of 45.0 and 5.77 × 10-5.
(2 marks)
3
ii. Derive a formula for the density, ρ (in lbm/ft ) above as a function of P (in psi).
(5 marks)
CLB 10904 CHEMICAL PROCESS PRINCIPLES 1
SEPTEMBER 2014 CONFIDENTIAL
iii. Calculate the density of the fluid (in lbm/ft3) if the pressure is 15.0 psi.
(2 marks)
(b) 0.5 kmol of ethane (C2H6) at 130C is placed in a 0.2 m 3 cylinder. Determine the
gauge pressure inside the cylinder using Soave-Redlich-Kwong equation of state
with the assumption C2H6 is a non-ideal gas and the atmospheric pressure as 1 atm.
(10 marks)
(c) Humid air at 88C and 1 atm with relative humidity of 95% is cooled to 27C at
constant pressure. Solve the dew point and degrees of superheat of the air at 88C.
(6 marks)
Question 3
A condenser is used to condense substances from gaseous to liquid state, typically by cooling it.
In this problem, a stream of humid air (58.0 mol% water) enters a condenser at 150C.
80% of the water vapor in the humid air is condensed and removed as pure liquid water.
Both gas and liquid phase streams leave the condenser at 30C. N2 gas leave the condenser
at the rate of 5.18 mol/s. Air consists of 21 mol% O2 and the balance N2.
(a) Draw and label a flowchart of the process.
(4 marks)
(b) Solve the total flow rate of the feed stream and both streams leaving the condenser.
(3 marks)
(c) Taking [N2 (g, 30C), O2 (g, 30C), and H2O (g, 30C)] as reference for enthalpy
calculations, prepare and fill in the inlet-outlet enthalpy table and calculate the heat
transferred to or from the condenser in kilowatts (Neglect the effects of pressure
changes on enthalpies).
(18 marks)
CLB 10904 CHEMICAL PROCESS PRINCIPLES 2
SEPTEMBER 2014 CONFIDENTIAL
Question 4
(a) In a large-scale steam methane (CH4) reforming plant, liquid H2O at 25C is first
evaporated at a rate of 1.50 m3/min. N2 gas is also feed to the evaporator at 25C
and absolute pressure of 250.0 kPa to avoid CH 4 combustion, which can be harmful.
The stream leaving the evaporator is mixed with a CH 4 stream before entering compressor.
The CH4 stream flows in at 25.0 m3/min at 25C and gauge pressure of 150.0 kPa.
The gases are subsequently compressed to a total absolute pressure of 500.0 kPa
at 200C and N2 must leave the compressor at an absolute pressure of 350.0 kPa.
In addition, the ratio of the molar flow rate of N 2 to H2O at the outlet stream leaving
the compressor is 5.0. The diagram of this evaporation-compression process is
shown below in Figure 2. Assume ideal gas behavior for the gas compounds and the
atmospheric pressure as 1 atm.
N2
Compressor
CH4
Evaporator
H2O H2O
N2
CH4
Figure 2
i. Solve the molar flow rate of H2O prior entering into the evaporator and molar
flow rate of CH4 prior entering into the compressor.
(6 marks)
ii. Calculate the molar composition (mol%) of H 2O, N2, and CH4 in the stream
leaving the compressor.
(6 marks)
iii. Determine the volumetric flow rate of N2 prior entering the evaporator.
(6 marks)
(b) A vapor mixture consist of 55 mol% 1-Propanol (C 3H8O) and 2-Propanol (C3H8O) is in
equilibrium with its liquid mixture at 70°C. The gas-liquid mixture exhibits ideal solution
behavior. Determine the system pressure and the composition of the liquid mixture.
(7 marks)
CLB 10904 CHEMICAL PROCESS PRINCIPLES 3
SEPTEMBER 2014 CONFIDENTIAL
Question 5
In the first step of the nitric acid production, ammonia (NH 3) must be oxidized with excess
air to form nitric oxide (NO) and H 2O. However, NH 3 oxidation also produces N 2 and H2O
in another reaction. 5000.0 kmol/hr of NH 3 enters the reactor at 25°C and 1 atm.
On the other hand, the air enters at 200°C and 1 atm at the rate of 6100.0 kmol/hr
(assume air consists of 21 mol% O2 and the balance N2). The gas mixtures exits the reactor
at 800°C and 1 atm with N 2 gas leaving the reactor at the rate of 5000.0 kmol/hr.
Selectivity of the desired, NO relative to the undesired, N 2 is 0.16.
4 NH3 ( g )+5 O 2 ( g )→4 NO ( g )+6 H 2 O ( v )
3
2 NH3 ( g )+ O 2 ( g )→N 2 ( g )+3 H 2 O ( v )
2
(a) Draw and label a flowchart of the process.
(3 marks)
(b) Solve the molar flow rate of NH 3 and O2 gases leaving the reactor using extent of
reaction method.
(7 marks)
(c) Taking elemental species [O2 (g), H2 (g), and N2 (g)] at 25°C and 1 atm as reference
for enthalpy calculations, fill in and complete the inlet-outlet enthalpy table below
(Table 1) and subsequently calculate the heat transferred to or from the reactor in
kilowatts.
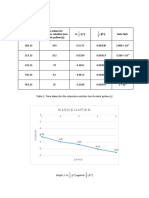
Table 1 Inlet-outlet enthalpy table
INPUT OUTPUT
Substance
nin (kmol/hr) Hin (kJ/mol) nout (kmol/hr) Hout (kJ/mol)
N2 (g) 5.13 23.86
O2 (g) 5.31 25.35
NH3 (g)
H2O (v)
NO (g)
(15 marks)
END OF QUESTION
CLB 10904 CHEMICAL PROCESS PRINCIPLES 4
SEPTEMBER 2014 CONFIDENTIAL
APPENDICES
A. List Of Formulations
ρi g kg lbm
1. SGi= ; ρref =1 . 000 3
=1000 . 0 3 =62 . 43 3
ρref cm m ft
1 x
2. M̄=∑ y i M i ; =∑ i
M̄ Mi
3 .n i=n io +νi ζ
Reactant input to process −Reactant output from process
4 . Overall Conversion= ×100 %
Reactant input to process
Reactant input to reactor −Reactant output from reactor
5 . Single-Pass Conversion= ×100 %
Reactant input to reactor
(nair , feed )−(nair ,theoretical ) n−n s
6 . % Excess Air= ×100 %; % Excess = ×100 %
(nair , theoretical ) ns
7 . Q̇−Ẇ s = Δ Ḣ+ Δ Ėk +Δ Ė p
T2
8 . Δ H=
^ ∫ C p (T )dT
T1
9 . Δ Ḣ=∑ ṅi H^ i −∑ ṅ i H^ i
out in
10 . ΔH =ξΔ H^ 0r + ∑ n out H^ out −∑ nin H^ in
out in
|n A , out−n A , in|
11. ξ=
|v A|
12. PV =nRT ; P V^ =zRT
V V^ Pc
13 . V^ = ;V ideal =
n r RT c
P T
14 . P r = ;T r =
Pc Tc
RT αa
The SRK equation of state: P= −
V^ −b V^ ( V^ +b)
( RT c )2 RT 2
a=0 . 42747 ;b=0 . 08664 c ;m=0. 48508+1 . 55171ω−0 . 1561ω 2 ;α=[ 1+m(1−√ T r ) ]
Pc Pc
P V^ B
The virial equation of state truncated after the second term: =1+
RT V^
RT c 0 . 422 0 . 172
B= (B o +ωB1 ); Bo =0. 083− ;B1 =0 . 139−
Pc T 1 .6 T 4 .2
r r
CLB 10904 CHEMICAL PROCESS PRINCIPLES 5
SEPTEMBER 2014 CONFIDENTIAL
¿
15 . pi = y i P= pi(T )
16 . pi = y i P= p¿i(T )
dewpoint
¿
17 . p A = y A P=x p A A(T )
18 . p A = y A P=x A H A(T )
p
19 . sr (hr )= i¿ ¿100%
pi( T )
B. Gas Constant
m3 . Pa. L . bar. L . atm L . mmHg
R=8. 314 =0 .08314 =0 . 08206 =62 .36
mol . K mol . K mol . K mol . K
3 3
ft . atm ft . psia
=0 .7302 =10 . 73
lb mol . o R lb mol . o R
cal J Btu
=1. 987 =8 .3144 =1 .987
mol . K mol . K lb mol . o R
C. Standard Conditions for Gases
System Ts Ps Vs ns
3
SI 273 K 1 atm 0.022415 m 1 mol
CGS 273 K 1 atm 22.415 L 1 mol
3
American Engineering 492˚R 1 atm 359.05 ft 1 Ib-mole
D. Pitzer Acentric Factors
Compound Acentric Factor, ω
Ammonia 0.250
Argon -0.004
Carbon dioxide 0.225
Carbon monoxide 0.049
Chlorine 0.073
Ethane 0.098
Hydrogen sulfide 0.100
Methane 0.008
Methanol 0.559
Nitrogen 0.040
Oxygen 0.021
Propane 0.152
Sulfur dioxide 0.251
Water 0.344
E. Factors For Unit Conversions
CLB 10904 CHEMICAL PROCESS PRINCIPLES 6
SEPTEMBER 2014 CONFIDENTIAL
Quantity Equivalent Values
Mass 1 kg = 1000 g = 0.001 metric ton = 2.20462 lbm = 35.27392 oz
1 lbm = 16 oz = 5 x 10–4 ton = 453.593 g = 0.453593 kg
Length 1m = 100 cm = 1000 mm = 106 microns (m) = 1010 angstroms (Å)
= 39.37 in = 3.2808 ft = 1.0936 yd = 0.0006214 mile
1 ft = 12 in = 1/3 yd = 0.3048 m = 30.48 cm
Volume 1 m3 = 1000 liters = 106 cm3 = 106 ml
= 35.3145 ft3 = 220.83 imperial gallons = 264.17 gal
= 1056.68 qt
1ft3 = 1728 in3 = 7.4805 gal = 0.028317 m3 = 28.317 liters
= 28 317 cm3
Force 1N = 1 kg.m/s2 = 105 dynes = 105 g.cm/s2 = 0.22481 lbf
1 lbf = 32.174 lbm.ft/s2 = 4.4482 N = 4.4482 x 105 dynes
Pressure 1 atm = 1.01325 x 105 N/m2 (Pa) = 101.325 kPa = 1.01325 bars
= 1.01325 x 106 dynes/cm2
= 760 mm Hg at 0C (torr) = 10.333 m H2O at 4C
= 14.696 lbf/in2 (psi) = 33.9 ft H2O at 4C
= 29.921 in Hg at 0C
Energy 1J = 1 N.m = 107 ergs = 107 dyne.cm
= 2.778 x 10-7 kW.h = 0.23901 cal
= 0.7376 ft-lbf = 9.486 x 10 - 4 Btu
Power 1W = 1 J/s = 0.23901 cal/s = 0.7376 ft.lbf/s = 9.486 x 10–4 Btu/s
= 1.341 x 10 - 3 hp
Temperature T(K) = T(C) + 273.15
T(F) = 1.8 T(C) + 32
T(R) = T(F) + 459.67
T(R) = 1.8 T(K)
CLB 10904 CHEMICAL PROCESS PRINCIPLES 7
You might also like
- Assignment CPP Jan 2020 PDFDocument5 pagesAssignment CPP Jan 2020 PDFNur Afifah IINo ratings yet
- KFT232 Sem2 2011 2012Document13 pagesKFT232 Sem2 2011 2012suhanizah suhanizahNo ratings yet
- 13 ME525 Practice Problems For Final Exam Partial Solutions 42313 PDFDocument7 pages13 ME525 Practice Problems For Final Exam Partial Solutions 42313 PDFalibaba011No ratings yet
- Sop Check Sheet: Maintenance Schedule and Checks/Tests of Transmission LinesDocument56 pagesSop Check Sheet: Maintenance Schedule and Checks/Tests of Transmission LinesAgusRiyantoNo ratings yet
- Assignment L01-T4Document8 pagesAssignment L01-T4MawareNo ratings yet
- CPP Assignment 1Document2 pagesCPP Assignment 1AmandaEdwinNo ratings yet
- Assignment L01 (Thursday, 11.30 Am)Document9 pagesAssignment L01 (Thursday, 11.30 Am)MawareNo ratings yet
- PC Question Paper Nov 2021Document4 pagesPC Question Paper Nov 2021venkatesan sivaramuNo ratings yet
- SCH 101HFN 141 Introduction To Physical ChemistryDocument4 pagesSCH 101HFN 141 Introduction To Physical Chemistryodib478No ratings yet
- Che 201 - Che Fundamentals Due Oct 7 (On-Campus) Due Oct 11 (Dedp)Document2 pagesChe 201 - Che Fundamentals Due Oct 7 (On-Campus) Due Oct 11 (Dedp)Andrew YauNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- CHM092 Tutorial Chapter 4ADocument8 pagesCHM092 Tutorial Chapter 4AvNo ratings yet
- r050210803 Chemical Process CalculationsDocument8 pagesr050210803 Chemical Process CalculationsSrinivasa Rao GNo ratings yet
- ChE 12 CHE 111 2014-15Document4 pagesChE 12 CHE 111 2014-15aanika roshniNo ratings yet
- Assignment FlexilearnDocument2 pagesAssignment Flexilearnfizo derisNo ratings yet
- 22315-2019-Winter-Question-Paper (Msbte Study Resources)Document4 pages22315-2019-Winter-Question-Paper (Msbte Study Resources)hollowpurple156No ratings yet
- CHE 312 Final Exam2013 - 2014 - RainDocument4 pagesCHE 312 Final Exam2013 - 2014 - RainChibuike CharlesNo ratings yet
- Sample Copy Test 1Document7 pagesSample Copy Test 1Louis LimNo ratings yet
- 2023 H2 Chemical Equilibria Tutorial (QP)Document15 pages2023 H2 Chemical Equilibria Tutorial (QP)nivind88No ratings yet
- Che 320 ExamDocument3 pagesChe 320 ExamAnjolaoluwa Oreoluwa AfolabiNo ratings yet
- 3 SEM/ Chemical Engineering / 2020 (W) NEW Th4 Industrial StoichiometryDocument2 pages3 SEM/ Chemical Engineering / 2020 (W) NEW Th4 Industrial StoichiometrySushanta K BeheraNo ratings yet
- Assignment L01 (Thursday, 11.30 Am) Marking SchemeDocument12 pagesAssignment L01 (Thursday, 11.30 Am) Marking SchemeMawareNo ratings yet
- Chemistry TutorialsDocument28 pagesChemistry TutorialsDomionNo ratings yet
- Revision STPM Term 1Document15 pagesRevision STPM Term 1Wong WengSiongNo ratings yet
- H2 Enrichment Paper 2 Chemical Eqm Gases and Atomic Structure (Ans)Document5 pagesH2 Enrichment Paper 2 Chemical Eqm Gases and Atomic Structure (Ans)Priya SivasubbramaniamNo ratings yet
- 2019 Summer Question Paper (Msbte Study Resources)Document4 pages2019 Summer Question Paper (Msbte Study Resources)hollowpurple156No ratings yet
- PC PDFDocument3 pagesPC PDFNeel PatelNo ratings yet
- 2021 August CH204-HDocument3 pages2021 August CH204-HMidhunNo ratings yet
- Assignment 1 2022Document5 pagesAssignment 1 2022Katlego NathanNo ratings yet
- W17 Process CalculationDocument4 pagesW17 Process CalculationAmey WankhedeNo ratings yet
- 2022 Summer Question Paper (Msbte Study Resources)Document4 pages2022 Summer Question Paper (Msbte Study Resources)hollowpurple156No ratings yet
- Exam 1 2014Document2 pagesExam 1 2014Diana BeirutiNo ratings yet
- Exercises NusDocument5 pagesExercises NusNor AzimahNo ratings yet
- Nr210803 Materials and Energy Balance Set1Document2 pagesNr210803 Materials and Energy Balance Set1Srinivasa Rao GNo ratings yet
- SCH 2102 Physical Chemistry IDocument4 pagesSCH 2102 Physical Chemistry ImutiganoahNo ratings yet
- GASES Free Response WorksheetDocument4 pagesGASES Free Response WorksheetJJNo ratings yet
- Practice FRQDocument4 pagesPractice FRQKrystal LiNo ratings yet
- SK015 KMPP Questions No AnswerDocument4 pagesSK015 KMPP Questions No AnswerRaudhatus NasuhaNo ratings yet
- Sheet 1Document2 pagesSheet 1Mohamed SalahNo ratings yet
- Chem 1Document3 pagesChem 1Lovey ChandiNo ratings yet
- CHE 307 (Thermodynamics)Document39 pagesCHE 307 (Thermodynamics)MdSiShovonNo ratings yet
- Chemistry SheetsDocument10 pagesChemistry Sheetshebaneyar74No ratings yet
- AL Chemistry 1995 Paper 1+2Document10 pagesAL Chemistry 1995 Paper 1+2api-3734333100% (1)
- Chang's Test Bank (Chapters 5, 7, 8, 9)Document27 pagesChang's Test Bank (Chapters 5, 7, 8, 9)asfaNo ratings yet
- Rr210803 Material Energy BalanceDocument8 pagesRr210803 Material Energy BalanceSrinivasa Rao G100% (2)
- Assignment 4Document3 pagesAssignment 4Duy Do MinhNo ratings yet
- AP Chemistry Fr3 Test BankDocument9 pagesAP Chemistry Fr3 Test BankzeustamNo ratings yet
- A Level Chemistry Paper 1 Set 31marking GuideDocument14 pagesA Level Chemistry Paper 1 Set 31marking GuidekitookebarnabasNo ratings yet
- Week 7 TutorialDocument4 pagesWeek 7 TutorialHua KhienNo ratings yet
- CH 2 Known Question TestDocument1 pageCH 2 Known Question TestFlick OPNo ratings yet
- Answer All Questions Part-A (5x2) (10 Marks) : V.SURESHKANNAN, AP II, MECH (Staff In-Charge)Document1 pageAnswer All Questions Part-A (5x2) (10 Marks) : V.SURESHKANNAN, AP II, MECH (Staff In-Charge)vsureshkannanmsecNo ratings yet
- Intro To Chemistry Unit: Exam RevisionDocument17 pagesIntro To Chemistry Unit: Exam RevisioncocoNo ratings yet
- Vakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6Document15 pagesVakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6vigiraneza0No ratings yet
- Chemicalprocesscalculations PDFDocument8 pagesChemicalprocesscalculations PDFSamiullah MohammedNo ratings yet
- Number of Printed Pages-6: EightDocument6 pagesNumber of Printed Pages-6: EightAmiya singhaNo ratings yet
- KC and KP Questions EquilibriaDocument8 pagesKC and KP Questions Equilibriakhadijaliyu3No ratings yet
- CPC 9Document8 pagesCPC 9rajaraghuramvarmaNo ratings yet
- 22315-2023-Summer-Question-Paper (Msbte Study Resources)Document4 pages22315-2023-Summer-Question-Paper (Msbte Study Resources)hollowpurple156No ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Chapter 1: BASIC BIOLOGY: Cell Theory Discovery of CellsDocument17 pagesChapter 1: BASIC BIOLOGY: Cell Theory Discovery of CellsMawareNo ratings yet
- Chap 2 BiochemDocument18 pagesChap 2 BiochemMawareNo ratings yet
- Experiment 3: Pulsed Column Liquid-Liquid Extraction UnitDocument13 pagesExperiment 3: Pulsed Column Liquid-Liquid Extraction UnitMawareNo ratings yet
- Callahan 2010Document8 pagesCallahan 2010MawareNo ratings yet
- Isothermal Compressibility of Aqueous Sodium Chloride, Magnesium Chloride, Sodium Sulfate, and Magnesium Sulfate Solutions From 0 To 45.deg. at 1 AtmDocument8 pagesIsothermal Compressibility of Aqueous Sodium Chloride, Magnesium Chloride, Sodium Sulfate, and Magnesium Sulfate Solutions From 0 To 45.deg. at 1 AtmMawareNo ratings yet
- Temperature (K) Time Taken For Colourless Solution Turn To Dark Yellow (S) in (S) (K) Rate M/sDocument4 pagesTemperature (K) Time Taken For Colourless Solution Turn To Dark Yellow (S) in (S) (K) Rate M/sMawareNo ratings yet
- Graph Thermo 4Document2 pagesGraph Thermo 4MawareNo ratings yet
- Universiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyDocument4 pagesUniversiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyMawareNo ratings yet
- Cover Page (Mini Project Submission Form)Document1 pageCover Page (Mini Project Submission Form)MawareNo ratings yet
- Summary 2. Introduction & Theories 3. Methodology 4. Results & Discussion 5. Conclusion & Recommendation 6. Tutorial 7. References and AppendicesDocument4 pagesSummary 2. Introduction & Theories 3. Methodology 4. Results & Discussion 5. Conclusion & Recommendation 6. Tutorial 7. References and AppendicesMawareNo ratings yet
- Test CKB 30103 ISH Part 1 QuestionDocument3 pagesTest CKB 30103 ISH Part 1 QuestionMawareNo ratings yet
- Multisim Is One of The Few Circuit Design Programs To Employ The Original Based Software SimulationDocument2 pagesMultisim Is One of The Few Circuit Design Programs To Employ The Original Based Software SimulationMawareNo ratings yet
- Tutorial 4 - FiltrationDocument4 pagesTutorial 4 - FiltrationMaware100% (1)
- Exp 2 Tray DryerDocument9 pagesExp 2 Tray DryerMawareNo ratings yet
- Assignment L01-T4Document8 pagesAssignment L01-T4MawareNo ratings yet
- Tutorial 10: 1. Integrate The Following Functions. A) B) C) D) E) F) H)Document4 pagesTutorial 10: 1. Integrate The Following Functions. A) B) C) D) E) F) H)MawareNo ratings yet
- Assignment L01 (Friday, 9.30) Marking SchemeDocument14 pagesAssignment L01 (Friday, 9.30) Marking SchemeMawareNo ratings yet
- Exp 3 Plate and Frame Filter PressDocument5 pagesExp 3 Plate and Frame Filter PressMawareNo ratings yet
- Assignment L01 (Thursday, 11.30 Am) Marking SchemeDocument12 pagesAssignment L01 (Thursday, 11.30 Am) Marking SchemeMawareNo ratings yet
- Assignment L01 (Thursday, 11.30 Am)Document9 pagesAssignment L01 (Thursday, 11.30 Am)MawareNo ratings yet
- Production of 70000 Mta Ethylene From MethaneDocument42 pagesProduction of 70000 Mta Ethylene From MethaneSyarif Wira'i100% (2)
- 07 - Saudi Kayan Mega ProjectDocument13 pages07 - Saudi Kayan Mega ProjectAbhimanyu SharmaNo ratings yet
- Edexcel AS Chemistry Unit 1 Jan 2013Document24 pagesEdexcel AS Chemistry Unit 1 Jan 2013Pakorn WinayanuwattikunNo ratings yet
- CONSTANTES TC PC VCDocument15 pagesCONSTANTES TC PC VCAngela tmNo ratings yet
- Seaborne EthaneDocument27 pagesSeaborne EthaneAnonymous icnhaNsFNo ratings yet
- Compressor ManualDocument411 pagesCompressor ManualJan75% (4)
- Gaseous Fuels: Application and Installation GuideDocument48 pagesGaseous Fuels: Application and Installation GuideDjebali MouradNo ratings yet
- Oil TransformerDocument5 pagesOil TransformerIndraNo ratings yet
- PetronasDocument30 pagesPetronasWoan Shiang100% (1)
- CoC - Marine Fuel OilsDocument64 pagesCoC - Marine Fuel OilsClarence ClarNo ratings yet
- Production of OlefinsDocument39 pagesProduction of OlefinsshubhamNo ratings yet
- Tabel All - Compressed 4Document92 pagesTabel All - Compressed 4Werdy PNo ratings yet
- Organic Geochemistry: Alexei V. Milkov, Giuseppe EtiopeDocument12 pagesOrganic Geochemistry: Alexei V. Milkov, Giuseppe EtiopeAngela GonzalezNo ratings yet
- Chemistry Class 10 Chapter 11Document14 pagesChemistry Class 10 Chapter 11Rahim BakhshNo ratings yet
- Production of FormaldehydeDocument21 pagesProduction of FormaldehydeU-sef Waleed100% (1)
- Demethanizer SimulationDocument31 pagesDemethanizer SimulationFranco Camacho CanchariNo ratings yet
- CIE IGCSE Chemistry Organic ADocument4 pagesCIE IGCSE Chemistry Organic AYee MeiNo ratings yet
- Ind. Eng. Chem. Prod. Res. Dev. Vol. 18 - No. 2 - 1979 135Document8 pagesInd. Eng. Chem. Prod. Res. Dev. Vol. 18 - No. 2 - 1979 135Lindsey BondNo ratings yet
- Iec60599 (Ed3 0) BDocument82 pagesIec60599 (Ed3 0) Bnamsaigon316No ratings yet
- Scope of Accreditation: Standards Council of Canada Conseil Canadien Des NormesDocument10 pagesScope of Accreditation: Standards Council of Canada Conseil Canadien Des NormeswandadwilestariNo ratings yet
- 6 ReCiPe111Document1,518 pages6 ReCiPe111Krishan AcharyaNo ratings yet
- Anic Chemistry 222-300Document12 pagesAnic Chemistry 222-300eamcetmaterialsNo ratings yet
- AGA5 Calculation-Imperial UnitDocument24 pagesAGA5 Calculation-Imperial UnitlubangjarumNo ratings yet
- Standard 3Document28 pagesStandard 3Muhammed SulfeekNo ratings yet
- Friends Boys School: Organic Chemistry SL / 12 IBDocument47 pagesFriends Boys School: Organic Chemistry SL / 12 IBKays Abu einNo ratings yet
- Hsslive XI Chemistry QB CH 13. HydrocarbonsDocument5 pagesHsslive XI Chemistry QB CH 13. Hydrocarbonsanumaria bijuNo ratings yet
- Adsorption of Methyl Orange Using Self-Assembled Porous MicrosphereDocument407 pagesAdsorption of Methyl Orange Using Self-Assembled Porous MicrosphereHaroon RashidNo ratings yet
- Reaction Kinetics of Ethane Partial Oxidation To Acetic AcidDocument10 pagesReaction Kinetics of Ethane Partial Oxidation To Acetic AcidAlejandro De la Rubia MarcosNo ratings yet
- Cambridge IGCSE: Chemistry 0620/22Document16 pagesCambridge IGCSE: Chemistry 0620/22lila davinci100% (1)