Professional Documents
Culture Documents
SilenCircle Complete RNAi Kit
SilenCircle Complete RNAi Kit
Uploaded by
AlleleBiotechOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SilenCircle Complete RNAi Kit
SilenCircle Complete RNAi Kit
Uploaded by
AlleleBiotechCopyright:
Available Formats
SilenCircleTM Complete RNAi
Expression Kit
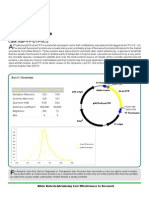
S ilenCircleTM RNAi system (pro-
tected under US patent 7,294,504
and additional patents pending) is
Box 1 | Product Summary
a plasmid-based RNA interference Catalogue Numbers ABP-RI-SC01010
system that uses engineered human ABP-RI-SC01020
U6 RNApolymerase III promoter and Components pre-cut pSIlenCircle Vector (10ng/µl)
modified terminator for high level pSilenCircle sequencing Primer (20µM)
small hairpin RNA (shRNA) or siRNA p53-top (coding for p53 RNAi insert top strand)
expression inside mammalian cells. (20µM)
The design enables precise start and p53-bot (coding for p53 RNAi insert bottom
end of an interfering RNA with the strand) (20µM)
optimal 3’ overhanging nucleotides. Annealing Buffer (10X)
The pre-cut linear vector is ready-to- hcT4 DNA Ligase with 10X Buffer
ligate for construction of interfering AvantGene
RNA expression plasmid with nearly DNA Diluent
zero self-ligation. Bearing a neomycin p53-5’ PCR Primer (20µM)
resistant marker, it may be used for p53-3’ PCR Primer (20µM)
establishing siRNA-expressing stable Actin-5’ PCR Primer (20µM)
cell lines. SilenCircle RNAi system Actin-3’ PCR Primer (20µM)
has been successfully used to knock- Storage Store the AvantGene at +4°C. All other rea-
down expression of both endogenous gents to be stored at -20°C.
and exogenous genes.
Stability All components are stable for 6 months when
Features stored properly.
T he SilenCircletm Complete RNAi
kit is suitable for RNAi experi-
ments in tissue culture cells. Each Reagents Provided with Design of Inserts
batch of reagents is vigorously tested
for consistency and stability, and offer the Kit
siRNA may be produced from two
the following features:
T he kit provides enough reagents pSilenCircle plasmids encoding either
- Efficient RNA interference to construct 10 RNAi expressing sense or antisense. Alternatively,
- Convenient ready-to-ligate format plasmids, including Allele recom- shRNA or miRNA may be produced
- Almost no background ligation binant high concentration T4 DNA from a single plasmid (shown below).
ligase (hcT4 DNA ligase). DNA oligos For each siRNA insert, two comple-
- Without introducing extra bases
encoding each RNAi target sequence mentary synthetic DNA oligos are
from restriction enzyme sites,
need to be designed according to the needed.
generate precise shRNA, siRNA, or
miRNA. guidelines listed below.
Choose a target region that is A2N19
Oligos for generating p53-specific (sense sequence of the target RNA),
Materials Not Suppplied design a linker sequence (e.g. 9
RNAi cassettes that have been tested
with the Kit to significantly reduce p53 mRNA bases), use the following format:
levels are also included as positive
control. 5’ acacc N19 Linker N’19 t 3’
-Target-specific insert DNA oligos 3’ g N’19 rcLinker N19 aaaaa 5’ #
-E. coli competent cells
The Complete Kit also provides
-Plasmid DNA purification system “N’19” is reverse/complementary to
AvantGeneTM transfection reagent,
-RNA purification system and reverse “N19”; “rclinker” is reverse/ comple-
suitable for most mammalian cells
transcription enzyme. mentary to “linker”.
with exceptional efficiency. RT-PCR
primers for detecting p53 and actin
# Oligos orders are typically entered
F or Research Use Only. Not for
Diagnostic or Therapeutic Use.
Purchase does not include or carry
mRNA levels are also included in the
Complete Kit. A successful p53 RNAi
from 5’ to 3’, i.e. aaaaa N19...
experiment is expected to result in Note: This product may be protected
any right to resell or transfer this reduced p53 mRNA levels while not
product either as a stand-alone under US patent 7,294,504 and additional
product or as a component of another
affecting actin mRNA levels. RT-PCR pending patents. Purchasing of this prod-
product. Any use of this product other with p53 primers should generate a uct grants the rights of use. Commercial
than the permitted use without the band of 496 bp; RT-PCR with actin user may be required to obtain further
express written authorization of Allele primers results in a band of 587b. license from third parties in order to use
certain RNA interference related technolo-
Biotech is strictly prohibited
gies.
Allele Biotech-Introducing Cost Effectiveness to Research
Protocols
Cloning Transfection
Oligo DNA annealing, ligation into the linearized vector, E. Cells are prepared and transfected generally as you
coli transformation, and plasmid DNA preparation may be would with a typical expression plasmid transfection. Most
performed according to standard protocols. Plasmid DNA commercial transfection reagents may be used with the
from positive clones will be cut only once by restriction SilenCircleTM plasmids. Although using AvantGene trans-
enzyme Stu I on the plasmid backbone (The Stu I site in fection reagent is recommended, in many cases the choice
the Polylinker should have been deleted from the pre-cut of transfection reagent should depend on cells to be used.
vector). A self-ligation plasmid (from the very few molecules Use 0.5 μg plasmid DNA per well of a 24-well plate as a
that escaped linearization of the vector) will yield two bands starting point.
of 1.1 kb and 3.5 kb, respectively. A sequencing primer is
also included in the kit for positive clone verification. Using AvantGene
Suggested Protocol for siRNA Insert Prepa- * All Volumes are for each well of 24-well plate:
ration
1. Plate cells approximately 24 hours prior to transfection
Top oligo 1μg at a cell density of 20-40% confluence in complete medium
(with serum and antibiotics if required).
Bottom Oligo 1μg
Annealing Buffer (10X) 2μl 2. Mix 2.5 μl AvantGene reagent to 10 μl serum free, antibi-
Distilled Water 20μl otics-free medium, incubate 5-10 min at room temperature.
Heat at 95°C for 10min, slowly cool down to room tempera- 3. Add 12.5 μl DNA Diluent to 0.5 μg DNA, incubate 1-5
ture. min at room temperature. Do not incubate longer than 5
min.
Suggested Protocol for DNA Ligation Using
Allele’s hcT4 DNA Ligase 4. While waiting, change cell medium to 200 μl serum-free
Pre-Cut Vector (ng/μl) 2μl and antibiotics-free medium.
Annealed insert 2μl 5. Mix diluted transfection reagent from Step 2 with DNA
Ligand buffer (10) 1μl solution from Step 3, incubate at room temperature for 5-10
T4 DNA ligase 0.5μl min.
Distilled water 4.5μl 6. Add the transfection mixture from step 5 drop-wise to
cells.
Incubate at room temperature for an hour or at 4-16°C
overnight. 7. After 2-4 hours incubation under appropriate condi-
tions in an incubator, add 250 μl serum-containing normal
medium.
Method Overview
Website: www.allelebiotech.com
Call: 1-800-991-RNAi/858-587-6645
(Pacific Time: 9:00AM~5:00PM)
Email: oligo@allelebiotech.com
Allele Biotech-Introducing Cost Effectiveness to Research
You might also like
- A0130765H - Lab Report 1 PDFDocument14 pagesA0130765H - Lab Report 1 PDFFionaNo ratings yet
- RNA Methodologies: A Laboratory Guide for Isolation and CharacterizationFrom EverandRNA Methodologies: A Laboratory Guide for Isolation and CharacterizationNo ratings yet
- 12 - Chemistry QP (Set-Ii)Document6 pages12 - Chemistry QP (Set-Ii)Shravan ZoneNo ratings yet
- Chapter 6 - Discrete MathDocument72 pagesChapter 6 - Discrete MathCharlesNo ratings yet
- SilenCircle Basic RNAi KitDocument2 pagesSilenCircle Basic RNAi KitAlleleBiotechNo ratings yet
- Line Silence RNAi Complete Expression KitDocument3 pagesLine Silence RNAi Complete Expression KitAlleleBiotechNo ratings yet
- LineSilence RNAi Mouse Expression KitDocument3 pagesLineSilence RNAi Mouse Expression KitAlleleBiotechNo ratings yet
- Synthetic Guide RNA For CRISPR Genome EditingDocument9 pagesSynthetic Guide RNA For CRISPR Genome EditinggiacummoNo ratings yet
- Package: Catalog No. SizeDocument3 pagesPackage: Catalog No. SizeHairul IslamNo ratings yet
- Adding HiBiT Tag To An Endogenous Gene Using CRISPR GE777Document4 pagesAdding HiBiT Tag To An Endogenous Gene Using CRISPR GE777Ram KishoreNo ratings yet
- Luciferase PCR Template For IVTDocument1 pageLuciferase PCR Template For IVTAlleleBiotechNo ratings yet
- Commercial Transfection Reagent ComparisonDocument9 pagesCommercial Transfection Reagent ComparisonA INo ratings yet
- CPNC 55Document12 pagesCPNC 55Amada El SabehNo ratings yet
- Rna Isolation and PurificationDocument62 pagesRna Isolation and PurificationBio BookNo ratings yet
- Recommended Checks and Controls For siRNA Experiments - AbcamDocument6 pagesRecommended Checks and Controls For siRNA Experiments - Abcamn7s77hxzbtNo ratings yet
- Module 7 - TECHNOLOGY and MTB - RIF Assay (Autosaved)Document70 pagesModule 7 - TECHNOLOGY and MTB - RIF Assay (Autosaved)Ermias Alemayehu BerisoNo ratings yet
- Convetional mRNA VaccineDocument1 pageConvetional mRNA VaccineKAIST이박터No ratings yet
- Rna Interference: PCR Strategies For The Quantification of Stable Degradation-Fragments Derived From Sirna-Targeted MrnasDocument5 pagesRna Interference: PCR Strategies For The Quantification of Stable Degradation-Fragments Derived From Sirna-Targeted Mrnasbiotecno1No ratings yet
- Quantitative Polymerase Chain ReactionDocument14 pagesQuantitative Polymerase Chain ReactionnavkirNo ratings yet
- Expression Vectors 1Document15 pagesExpression Vectors 1keyonthewebNo ratings yet
- Chapter 5Document32 pagesChapter 5bmwk1200rNo ratings yet
- Expression ProfilingDocument8 pagesExpression ProfilingSreedurgalakshmi KNo ratings yet
- Inverse Polymerase Chain Reaction (Inverse PCR) Is A Variant of TheDocument8 pagesInverse Polymerase Chain Reaction (Inverse PCR) Is A Variant of TheNiraj Agarwal100% (1)
- Polymerase Chain ReactionDocument36 pagesPolymerase Chain ReactionRajeswariNo ratings yet
- RNA Purification Kit Comparison: Yield, Quality and Real-Time RT-PCR PerformanceDocument7 pagesRNA Purification Kit Comparison: Yield, Quality and Real-Time RT-PCR PerformanceLinbert Simon CallataNo ratings yet
- Polymerase Chain ReactionDocument35 pagesPolymerase Chain ReactionVenakadalakshmi Kannappan100% (1)
- Chapter 13 - Molecular MethodsDocument29 pagesChapter 13 - Molecular MethodsYoiceMartinaPawekaNo ratings yet
- Description: Sensifast™ Cdna Synthesis KitDocument2 pagesDescription: Sensifast™ Cdna Synthesis KitDevin HendrawanNo ratings yet
- Origin and HistoryDocument3 pagesOrigin and HistoryDeidrae OuanoNo ratings yet
- DNA Recombination HandoutDocument1 pageDNA Recombination HandoutAllen Jierqs SanchezNo ratings yet
- Park Et Al. 2014Document8 pagesPark Et Al. 2014Benedikt EngelNo ratings yet
- Netic Code and Gene Expression (Transcription) - PT 1 - 08.10.21 - For UploadDocument34 pagesNetic Code and Gene Expression (Transcription) - PT 1 - 08.10.21 - For UploadPeaches HagleyNo ratings yet
- Cha RS y Thilly WG 1993Document13 pagesCha RS y Thilly WG 1993Marcelino SoteloNo ratings yet
- HB-2433-002 HB miRC LNA RNA Spike-In RT 0420 WWDocument24 pagesHB-2433-002 HB miRC LNA RNA Spike-In RT 0420 WWabha.kush28No ratings yet
- In Situ Hybridization: Lab of Neural Development IONDocument45 pagesIn Situ Hybridization: Lab of Neural Development IONapi-3701422No ratings yet
- Hb-2830-001 HB Qiaprepamp Viral Rna Um 1120 WWDocument40 pagesHb-2830-001 HB Qiaprepamp Viral Rna Um 1120 WWNguyen QuanNo ratings yet
- RNA Sequencing: An Introduction To Efficient Planning and Execution of RNA Sequencing (RNA-Seq) ExperimentsDocument6 pagesRNA Sequencing: An Introduction To Efficient Planning and Execution of RNA Sequencing (RNA-Seq) ExperimentsnareshNo ratings yet
- Lecture 1Document38 pagesLecture 1Đức Huy NguyễnNo ratings yet
- Method and Result:: 2.1. Sg-RNA DesigningDocument9 pagesMethod and Result:: 2.1. Sg-RNA DesigningMasum Billah TuhinNo ratings yet
- QPCR SopDocument7 pagesQPCR SopAna Marina SantosNo ratings yet
- Laboratorio Biologia MolecolareDocument250 pagesLaboratorio Biologia MolecolareCarIo Alberto Sca0% (1)
- RNA Synthesis, Processing & ModificationDocument53 pagesRNA Synthesis, Processing & Modificationamanialwerfalli4No ratings yet
- Optimization of Transfection Conditions and Analysis of Sirna Potency Using Real-Time PCRDocument15 pagesOptimization of Transfection Conditions and Analysis of Sirna Potency Using Real-Time PCRapi-117586719No ratings yet
- COVID-19 - Real Time RNA: 50 / 100 /150 Tests (Ready To Use Kit)Document5 pagesCOVID-19 - Real Time RNA: 50 / 100 /150 Tests (Ready To Use Kit)lupibudiNo ratings yet
- Cms 056069Document12 pagesCms 056069nitu thulasiNo ratings yet
- Gene ExpressionDocument29 pagesGene Expressionomar khaled العكلNo ratings yet
- Genome Editing: PLNT2530 (2020) Unit 9Document25 pagesGenome Editing: PLNT2530 (2020) Unit 9Swati JainNo ratings yet
- RT-PCR Two-Steps ProtocolDocument13 pagesRT-PCR Two-Steps ProtocolFrancisco MartinezNo ratings yet
- PCR MultiplexDocument2 pagesPCR MultiplexFatima VessaliusNo ratings yet
- Developing mRNA-vaccine Technologies: RNA BiologyDocument13 pagesDeveloping mRNA-vaccine Technologies: RNA BiologytranscriptasareversaNo ratings yet
- Genetics Engineering PPT Grp#09Document52 pagesGenetics Engineering PPT Grp#09Alina RajputNo ratings yet
- MeV KIT HMV PDFDocument12 pagesMeV KIT HMV PDFSeby SebastianNo ratings yet
- Tilapa Lake Virus One-Step ManualDocument5 pagesTilapa Lake Virus One-Step ManualCuong NguyenNo ratings yet
- Lecture 3 4Document140 pagesLecture 3 4ngocnm.bi12-320No ratings yet
- Introduction To Molecular BiologyDocument23 pagesIntroduction To Molecular Biologykris_bt20029241No ratings yet
- Restriction Enzyme (Restriction Endonuclease)Document18 pagesRestriction Enzyme (Restriction Endonuclease)yusuf zihniNo ratings yet
- Assignment Namin Ni MaderDocument6 pagesAssignment Namin Ni MaderGervy Racoma ValerosoNo ratings yet
- Advanced PCR: Methods and Applications: Dr. Maryke AppelDocument21 pagesAdvanced PCR: Methods and Applications: Dr. Maryke AppelDeepak RohithNo ratings yet
- Micro and Biotech FPDocument12 pagesMicro and Biotech FPmukul sidhqueNo ratings yet
- Santillan 2017Document11 pagesSantillan 2017Miguel RamirezNo ratings yet
- DNA To RNA To Protein-66587207Document40 pagesDNA To RNA To Protein-66587207Abdo HusseinNo ratings yet
- Fast Facts: Comprehensive Genomic Profiling: Making precision medicine possibleFrom EverandFast Facts: Comprehensive Genomic Profiling: Making precision medicine possibleNo ratings yet
- High Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- Modified UTPDocument1 pageModified UTPAlleleBiotechNo ratings yet
- Modified CTPDocument1 pageModified CTPAlleleBiotechNo ratings yet
- M13KO7 Helper PhageDocument1 pageM13KO7 Helper PhageAlleleBiotechNo ratings yet
- Anti-RFP (3F5) Monoclonal AntibodyDocument1 pageAnti-RFP (3F5) Monoclonal AntibodyAlleleBiotechNo ratings yet
- High Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- Anti-RFP (5F8) Monoclonal AntibodyDocument1 pageAnti-RFP (5F8) Monoclonal AntibodyAlleleBiotechNo ratings yet
- PNCS dLanYFPDocument1 pagePNCS dLanYFPAlleleBiotechNo ratings yet
- Custom Sub Cloning ServiceDocument1 pageCustom Sub Cloning ServiceAlleleBiotechNo ratings yet
- Mwasabi PCR Template For IVTDocument1 pageMwasabi PCR Template For IVTAlleleBiotechNo ratings yet
- Allele AgaroseDocument1 pageAllele AgaroseAlleleBiotechNo ratings yet
- mClavGR2 Fusion VectorsDocument3 pagesmClavGR2 Fusion VectorsAlleleBiotechNo ratings yet
- pmTFP1 ClathrinDocument4 pagespmTFP1 ClathrinAlleleBiotechNo ratings yet
- 3'-Amino-Modifier C7 CPGDocument1 page3'-Amino-Modifier C7 CPGAlleleBiotechNo ratings yet
- ChromoTek RFP BoosterDocument1 pageChromoTek RFP BoosterAlleleBiotechNo ratings yet
- Luciferase PCR Template For IVTDocument1 pageLuciferase PCR Template For IVTAlleleBiotechNo ratings yet
- 3' DG-CPGDocument1 page3' DG-CPGAlleleBiotechNo ratings yet
- 3' DT-CPGDocument1 page3' DT-CPGAlleleBiotechNo ratings yet
- 3'-Thiol-Modifier C6 S-S CPGDocument1 page3'-Thiol-Modifier C6 S-S CPGAlleleBiotechNo ratings yet
- 3' DC-CPGDocument1 page3' DC-CPGAlleleBiotechNo ratings yet
- Gryphon Selection MediumDocument1 pageGryphon Selection MediumAlleleBiotechNo ratings yet
- Lesson Plan FinalDocument4 pagesLesson Plan Finalapi-242797673No ratings yet
- Ch4 TranscriptionDocument10 pagesCh4 Transcriptionlina taziNo ratings yet
- Category 2-Bonus PacketDocument9 pagesCategory 2-Bonus Packetapi-312542882No ratings yet
- Next Generation Sequencing - : An OverviewDocument46 pagesNext Generation Sequencing - : An OverviewShuaib AhmadNo ratings yet
- Syllabus BSCBOTDocument18 pagesSyllabus BSCBOTJayanta SikdarNo ratings yet
- Biology Topic Guide EpigeneticsDocument19 pagesBiology Topic Guide EpigeneticsGazar100% (2)
- Activity1 Dela CernaDocument4 pagesActivity1 Dela CernaSamantha Dela CernaNo ratings yet
- Course Outline 2016-2017: St. Mary'S College Cape Biology Unit 1Document3 pagesCourse Outline 2016-2017: St. Mary'S College Cape Biology Unit 1Osmany MadrigalNo ratings yet
- Dna Sequencing by Sanger MethodDocument19 pagesDna Sequencing by Sanger MethodSethu SKNo ratings yet
- 149 276 1 SM PDFDocument5 pages149 276 1 SM PDFDiana AjahNo ratings yet
- NEET 2021, Download The Previous Year NEET Question Paper With The Answer Key For The YearsDocument20 pagesNEET 2021, Download The Previous Year NEET Question Paper With The Answer Key For The YearsZephyr EntranceNo ratings yet
- BCH 305 (Chemistry & Metabolism of Nucleic Acids)Document30 pagesBCH 305 (Chemistry & Metabolism of Nucleic Acids)ihebunnaogochukwu24No ratings yet
- QIAamp DNA Mini Blood MiniDocument72 pagesQIAamp DNA Mini Blood MiniYoNo ratings yet
- IBDP Biology Revision Guide (SL) - Knowledge and ApplicationDocument43 pagesIBDP Biology Revision Guide (SL) - Knowledge and Application[5L14] Cheung Samara Nathania100% (1)
- General MicrobiologyDocument68 pagesGeneral MicrobiologySupriya SharmaNo ratings yet
- Chapter 1 - Exploring LifeDocument69 pagesChapter 1 - Exploring LifeNursyakilaNo ratings yet
- Basic Laboratory Protocol Guide IN Molecular Biology and BiotechnologyDocument4 pagesBasic Laboratory Protocol Guide IN Molecular Biology and BiotechnologyMain Sanatani HunNo ratings yet
- Genetic Engineering 2023Document12 pagesGenetic Engineering 2023Nashawn BrownNo ratings yet
- Replicacion ADNDocument2 pagesReplicacion ADNSebastian NeculqueoNo ratings yet
- Module of BiotechnologyDocument69 pagesModule of BiotechnologyNisha HaldarNo ratings yet
- Biodiversity: (Prices Are Subject To Change Without Notice)Document52 pagesBiodiversity: (Prices Are Subject To Change Without Notice)Paras PathakNo ratings yet
- MCQ BiologyDocument3 pagesMCQ BiologyThayumanavanNo ratings yet
- Molecular BiotechnologyDocument40 pagesMolecular BiotechnologyBhaskar GangulyNo ratings yet
- Examkrackers Lecture 2 Section QuestionsDocument3 pagesExamkrackers Lecture 2 Section QuestionsAyodejiES1No ratings yet
- Entrepreneurial DNADocument6 pagesEntrepreneurial DNADylan GregerNo ratings yet
- The Cell NucleusDocument3 pagesThe Cell NucleushutterincNo ratings yet
- Teachable Tidbit Developed For The: SMI Scientific Teaching InstituteDocument14 pagesTeachable Tidbit Developed For The: SMI Scientific Teaching InstitutePelli BridgertonNo ratings yet