Professional Documents
Culture Documents
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
Uploaded by
Grace HernandezCopyright:
Available Formats
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Usp Description and SolubilityDocument1 pageUsp Description and SolubilityvafaashkNo ratings yet
- Print Expt7 Lab ReportDocument7 pagesPrint Expt7 Lab ReportShaliza Hernandez100% (2)
- CottonDocument16 pagesCottonKushagradhi DebnathNo ratings yet
- PHA6111 Lab ACTIVITY 2 HERNANDEZDocument4 pagesPHA6111 Lab ACTIVITY 2 HERNANDEZGrace HernandezNo ratings yet
- Wiki Anal Tit Nonaqueous TitrationDocument5 pagesWiki Anal Tit Nonaqueous TitrationMira JunitaNo ratings yet
- Lab Manual Writeup - Flower Liq-Liq SeparationDocument10 pagesLab Manual Writeup - Flower Liq-Liq SeparationNiharika MalikNo ratings yet
- 5350-5354 Reference Tables - Description and Solubility - ADocument5 pages5350-5354 Reference Tables - Description and Solubility - Apate malabananNo ratings yet
- Descrição e SolubilidadeDocument71 pagesDescrição e Solubilidadereg.analista10No ratings yet
- PHA615 LAB Experiment 4Document3 pagesPHA615 LAB Experiment 4POMPEYO BARROGANo ratings yet
- Chemistry, Water, and Buffers M123Document35 pagesChemistry, Water, and Buffers M123A-Naeem To'mah Al-sawaieNo ratings yet
- Post Lab NotesDocument18 pagesPost Lab Notesshervintancruzado91% (11)
- Lab Manual NÂ3 2023 Acid Base TitrationDocument5 pagesLab Manual NÂ3 2023 Acid Base TitrationabderraouftabaniNo ratings yet
- Acyl CompoundsDocument35 pagesAcyl Compoundsami tiuNo ratings yet
- Chapter 19 Acids and Bases PPT Glembocki 2017Document51 pagesChapter 19 Acids and Bases PPT Glembocki 2017niaNo ratings yet
- 3 - Acid Base Titration in Nonaqueous - DSWDocument27 pages3 - Acid Base Titration in Nonaqueous - DSWbrianNo ratings yet
- USP 42 Description & Relative SolubilityDocument85 pagesUSP 42 Description & Relative Solubilityirhamfauzzi100% (3)
- Jimin - Lesson19.2C (J)Document8 pagesJimin - Lesson19.2C (J)ClaraNo ratings yet
- Non Aqueous TitrationkkkDocument7 pagesNon Aqueous Titrationkkksatheeshpharma6No ratings yet
- Acid-Base Titration in Nonaqueous SolventsDocument6 pagesAcid-Base Titration in Nonaqueous Solventsliz_hobbs79No ratings yet
- Properties of Organic Compounds With Carbonyl GroupDocument14 pagesProperties of Organic Compounds With Carbonyl GroupnadyahginiceNo ratings yet
- Lesson - Acids, Alkalis, and NeutralisationDocument21 pagesLesson - Acids, Alkalis, and NeutralisationĐỗ NguyênNo ratings yet
- Physical Properties of Organic Compounds: Mr. Maywan HarionoDocument27 pagesPhysical Properties of Organic Compounds: Mr. Maywan HarionoJudy MelegritoNo ratings yet
- Acid and Base ReactionsDocument4 pagesAcid and Base ReactionsAntoline Natasha RayappanNo ratings yet
- FTIR Infrared Spectroscopy Absorption TableDocument4 pagesFTIR Infrared Spectroscopy Absorption TablePankaj Kumar PalNo ratings yet
- Org Chem Ost LabDocument21 pagesOrg Chem Ost Labjullian marasiganNo ratings yet
- Neutralization PrelimsDocument3 pagesNeutralization PrelimsJan Paolo TabbalNo ratings yet
- 08 - Infrared Spectroscopy ManualDocument5 pages08 - Infrared Spectroscopy ManualShubham BobadeNo ratings yet
- Dyes & Color by Mansoor IqbalDocument8 pagesDyes & Color by Mansoor Iqbalmansoor iqbal100% (4)
- Acids and Bases: PGCC CHM 101 SinexDocument24 pagesAcids and Bases: PGCC CHM 101 SinexRisna YusniNo ratings yet
- Acids and Bases: PGCC CHM 101 SinexDocument24 pagesAcids and Bases: PGCC CHM 101 Sinex7-SAL 2022No ratings yet
- ف-2-1 2Document18 pagesف-2-1 2Noor FarhanNo ratings yet
- Chem 4Document103 pagesChem 4César Arenas100% (1)
- Non Aqeuous TitrationDocument7 pagesNon Aqeuous Titrationsurabhi tadeNo ratings yet
- BCH 303 Lecture 1Document20 pagesBCH 303 Lecture 1tyhana24No ratings yet
- Solutions 2Document29 pagesSolutions 2Lawrence CarlNo ratings yet
- Acids and BasesDocument5 pagesAcids and BasesLidia CavalieriNo ratings yet
- Acid and Alkali Worksheet 2Document3 pagesAcid and Alkali Worksheet 2Shafiqah Airadz100% (1)
- Acids and Bases: Regents: Chapter 19 P. 586 - 629 Honors: Chapters 20, 21 P. 576 - 643Document103 pagesAcids and Bases: Regents: Chapter 19 P. 586 - 629 Honors: Chapters 20, 21 P. 576 - 643Zaina ZaliraNo ratings yet
- Welcome To My PresentationDocument17 pagesWelcome To My PresentationMrym NbNo ratings yet
- Hsslive Xii Chemistry Lab VOLUMETRIC ANALYSIS TitrationsDocument16 pagesHsslive Xii Chemistry Lab VOLUMETRIC ANALYSIS Titrationsmalavikasunil107No ratings yet
- Acid & Bases 11.1-11.3Document42 pagesAcid & Bases 11.1-11.3harshvaardhanNo ratings yet
- Acid and BaseDocument20 pagesAcid and BaseChris MaNo ratings yet
- Investigatory Project Chemistry PH in Everyday LifeDocument23 pagesInvestigatory Project Chemistry PH in Everyday LifeHarshita Jain67% (3)
- Fedor Kudriavtcev - iGCSE - Chem - Worksheet 16 - Acids, Alkalis & TitrationDocument2 pagesFedor Kudriavtcev - iGCSE - Chem - Worksheet 16 - Acids, Alkalis & Titrationyeet0624kfd0624No ratings yet
- Non Aqueous TitrationDocument31 pagesNon Aqueous TitrationRavi KaushikNo ratings yet
- Acid and BasesDocument27 pagesAcid and BasesdaphneleixiNo ratings yet
- Acids and BasesDocument5 pagesAcids and BasesMuniel Yuri NazarNo ratings yet
- Acid and Alkalis Glossary-PosterDocument1 pageAcid and Alkalis Glossary-PosterEuriz RamosNo ratings yet
- Acids, Bases & BuffersDocument8 pagesAcids, Bases & BuffersChetan JainNo ratings yet
- Water: The Solvent For Biochemical ReactionsDocument53 pagesWater: The Solvent For Biochemical ReactionssarahyahayaNo ratings yet
- Acids, Bases and Salts: Experiment 7Document15 pagesAcids, Bases and Salts: Experiment 7asfass sfasfasfasNo ratings yet
- YT Acid Base and Salt 1Document77 pagesYT Acid Base and Salt 1reyanshNo ratings yet
- Act1 2Section1DRiboDelDocument15 pagesAct1 2Section1DRiboDelRIBO, DELNo ratings yet
- Indicators ExperimentDocument3 pagesIndicators Experimentdhruv.sachdevaNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Pigments, Paint and Painting: A practical book for practical menFrom EverandPigments, Paint and Painting: A practical book for practical menNo ratings yet
- Autonomic Nervous System Drugs: Endogenous AdrenergicsDocument23 pagesAutonomic Nervous System Drugs: Endogenous AdrenergicsGrace HernandezNo ratings yet
- Polyfunctional Prac SetDocument6 pagesPolyfunctional Prac SetGrace Hernandez100% (1)
- Experiment 2: Postlab Qualitative Tests For Proteins RecallDocument15 pagesExperiment 2: Postlab Qualitative Tests For Proteins RecallGrace HernandezNo ratings yet
- HERNANDEZ Assign3 PDFDocument3 pagesHERNANDEZ Assign3 PDFGrace HernandezNo ratings yet
- Phyphar Lab First ShiftingDocument5 pagesPhyphar Lab First ShiftingGrace HernandezNo ratings yet
- Dispensing 1 PrescriptionDocument2 pagesDispensing 1 PrescriptionGrace HernandezNo ratings yet
- Hernandez Theo Summative ExamDocument1 pageHernandez Theo Summative ExamGrace HernandezNo ratings yet
- Hernandez, Grace R. 2A-PH Assignment No. 2Document3 pagesHernandez, Grace R. 2A-PH Assignment No. 2Grace HernandezNo ratings yet
- Hernandez, Grace R. 2A-PH Assignment No. 1Document3 pagesHernandez, Grace R. 2A-PH Assignment No. 1Grace HernandezNo ratings yet
- Ficia L: Erythromycin OintmentDocument2 pagesFicia L: Erythromycin OintmentGrace HernandezNo ratings yet
- (SANTOS) Part 1 Unit 5 FINANCIAL MANAGEMENTDocument34 pages(SANTOS) Part 1 Unit 5 FINANCIAL MANAGEMENTGrace HernandezNo ratings yet
- Rizal Readings 1Document5 pagesRizal Readings 1Grace HernandezNo ratings yet
- Dispensing Lec Activity 1 and 2Document2 pagesDispensing Lec Activity 1 and 2Grace HernandezNo ratings yet
- Micropara Lec Unit 1 LaDocument1 pageMicropara Lec Unit 1 LaGrace HernandezNo ratings yet
- USP NF HydrocortisoneDocument2 pagesUSP NF HydrocortisoneGrace HernandezNo ratings yet
- USP NF NystatinDocument1 pageUSP NF NystatinGrace HernandezNo ratings yet
- INORG LAB Reactions of Alkali GroupDocument1 pageINORG LAB Reactions of Alkali GroupGrace HernandezNo ratings yet
- Virus: Clostridium Tetani - Cause TetanusDocument1 pageVirus: Clostridium Tetani - Cause TetanusGrace HernandezNo ratings yet
- Fundamental Features of Microbiology: VirusesDocument21 pagesFundamental Features of Microbiology: VirusesGrace HernandezNo ratings yet
- Case Application For Decision-Making PDFDocument2 pagesCase Application For Decision-Making PDFGrace HernandezNo ratings yet
- GRH - 1aph - 1Document9 pagesGRH - 1aph - 1Grace HernandezNo ratings yet
- Performance Task in Physics: University of Santo TomasDocument4 pagesPerformance Task in Physics: University of Santo TomasGrace HernandezNo ratings yet
- Participatory Capacities and Vulnerabilities Assessment Part 1Document5 pagesParticipatory Capacities and Vulnerabilities Assessment Part 1Grace HernandezNo ratings yet
- Course Plan 2019 - Pha 617 (Pharmacy Administration, Leadership, and Management)Document14 pagesCourse Plan 2019 - Pha 617 (Pharmacy Administration, Leadership, and Management)Grace Hernandez100% (1)
- Saint Agnes Parish: Evangelization and Service To OthersDocument3 pagesSaint Agnes Parish: Evangelization and Service To OthersGrace HernandezNo ratings yet
- Pha619 Lec Chap 2Document9 pagesPha619 Lec Chap 2Grace HernandezNo ratings yet
- TissueDocument1 pageTissueGrace HernandezNo ratings yet
- PHA618 - Anatomical TermsDocument4 pagesPHA618 - Anatomical TermsGrace Hernandez100% (1)
- Chem 31.1 Postlab 9Document1 pageChem 31.1 Postlab 9Sellina SyNo ratings yet
- List of Hazardous Chemical As Per Notification Dated 19 TH January 2000Document18 pagesList of Hazardous Chemical As Per Notification Dated 19 TH January 2000saikumar selaNo ratings yet
- Aluminium NitrateDocument3 pagesAluminium NitrategumtammNo ratings yet
- RA Sir JEE PYQ Organic ChemistryDocument191 pagesRA Sir JEE PYQ Organic Chemistrydash gupta100% (4)
- Organic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusDocument6 pagesOrganic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusMadison FullerNo ratings yet
- Chemical Composition of PetroleumDocument18 pagesChemical Composition of PetroleumVeera Reddy BijjaramNo ratings yet
- SALTINGDocument6 pagesSALTINGGret ChenNo ratings yet
- Follow Up Sheet GUDocument26 pagesFollow Up Sheet GUnomanNo ratings yet
- C Family Silicon, Silicates and Their TypesDocument6 pagesC Family Silicon, Silicates and Their TypesUsman GhaniNo ratings yet
- Ammonium GlycyrrhizateDocument2 pagesAmmonium GlycyrrhizateAndré C. de PaulaNo ratings yet
- Physical Pharmacy - Second Stage Lecture 9Document82 pagesPhysical Pharmacy - Second Stage Lecture 9atheeralmadridi48No ratings yet
- Sintesis de IndolesDocument14 pagesSintesis de IndolesLili AmadorNo ratings yet
- MATHEMATICS - (12th & 13th) Paper-2Document3 pagesMATHEMATICS - (12th & 13th) Paper-2Raju SinghNo ratings yet
- June 2020 (R) QPDocument28 pagesJune 2020 (R) QPafzalafsaNo ratings yet
- Food Chemistry Volume 128 Issue 3 2011 (Doi 10.1016 - J.foodchem.2011.03.075) He-Ya Wang He Qian Wei-Rong Yao - Melanoidins P PDFDocument12 pagesFood Chemistry Volume 128 Issue 3 2011 (Doi 10.1016 - J.foodchem.2011.03.075) He-Ya Wang He Qian Wei-Rong Yao - Melanoidins P PDFOktavia Rahayu ANo ratings yet
- Reaction Mechanism Worksheet PDFDocument60 pagesReaction Mechanism Worksheet PDFSacchitDSheth67% (3)
- Chemistry Revision Worksheet CH1Document16 pagesChemistry Revision Worksheet CH1gcubeyyNo ratings yet
- Prediksi NamaDocument44 pagesPrediksi NamaNurlailatush SholihahNo ratings yet
- Class 12 CBSE Electrochemistry QuestionsDocument1 pageClass 12 CBSE Electrochemistry Questionsankitsingh90No ratings yet
- Titration EssentialsDocument87 pagesTitration EssentialsFabio GreenNo ratings yet
- EdExcel A Level Chemistry Unit 7 Mark Scheme Jan 2000Document4 pagesEdExcel A Level Chemistry Unit 7 Mark Scheme Jan 2000MashiatUddinNo ratings yet
- Eng Chem Lecture NotesDocument2 pagesEng Chem Lecture NotesJunell TadinaNo ratings yet
- Aqa Chemistry Jan 2011 Past Paper PDFDocument16 pagesAqa Chemistry Jan 2011 Past Paper PDFTiffany MaddoxNo ratings yet
- Trouble Shooting in Dyeing ProcessDocument26 pagesTrouble Shooting in Dyeing ProcessAnonymous wA6NGuyklDNo ratings yet
- Shell HD Premium Coolant N Pre Diluted 50 - 50Document1 pageShell HD Premium Coolant N Pre Diluted 50 - 50Binar KusumahNo ratings yet
- Chem ReportDocument10 pagesChem Reportzyan himorNo ratings yet
- Chem 1Document111 pagesChem 1C1A 05 Ashwina JNo ratings yet
- List ASTM For Water Testing StandardsDocument13 pagesList ASTM For Water Testing StandardsAnonymous a19X9GHZNo ratings yet
- Etherificacion Del GlicerolDocument8 pagesEtherificacion Del GlicerolGastón Miranda GarcíaNo ratings yet
- Hemsheela Model School Durgapur Term-Ii Examination-2022 Chemistry Marking Scheme Class-XiDocument6 pagesHemsheela Model School Durgapur Term-Ii Examination-2022 Chemistry Marking Scheme Class-XiBaichitra MondalNo ratings yet
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
Uploaded by
Grace HernandezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
EXPERIMENT 6: Comparative Investigation of Organic Compounds Prelab Activity (Individual) NAME: Grace R. Hernandez - SECTION:1A-PH
Uploaded by
Grace HernandezCopyright:
Available Formats
EXPERIMENT 6: Comparative Investigation of Organic Compounds
PRELAB ACTIVITY (INDIVIDUAL)
NAME: Grace R. Hernandez_________________________________ SECTION:1A-PH________________
Given the statement about alcohol, write its properties per column.
Ethanol is a volatile, colorless liquid that has a slight odor. It burns with a smokeless blue flame
that is not always visible in normal light. It is slightly more refractive than water, having a refractive
index of 1.36242. It is miscible with water and with many organic solvents. In the presence of acid
catalysts, ethanol reacts with carboxylic acids to produce ethyl esters and water. Strong acid desiccants
cause the dehydration of ethanol to form ethane or diethyl ether. Complete combustion of ethanol
forms carbon dioxide and water vapor. Ethanol can be oxidized to acetaldehyde and further oxidized
to acetic acid, depending on the reagents and conditions.
PROPERTIES OF ETHANOL
PHYSICAL PROPERTIES CHEMICAL PROPERTIES
Volatile Reacts with carboxylic acids in the presence
of acid catalysts to produce ethyl esters and
water
Colorless liquid Dehydrates to form ethane or diethyl ether
with strong acid desiccants
Has a slight odor Forms carbon dioxide and water vapor in its
complete combustion
Highly flammable Can be oxidized to acetaldehyde
Burns with a smokeless blue flame that is not Further oxidation will form acetic acid
always visible in normal light
Slightly more refractive than water
Has a refractive index of 1.36242
Miscible with water and with many organic
solvents
*You may add more rows if the rows are not enough.
PHA 615 LABORATORY EXPERIMENT 6 | Page 1 of 4
EXPERIMENT 6: Comparative Investigation of Organic Compounds
DRY LAB
NAME: Grace R. Hernandez_________________________________ SECTION:1A-PH________________
Predict the properties of the given compounds. Fill the table based on the discussion.
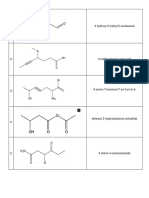
Cyclohexan Benzoic Ethyl Ethylamin
Test compounds e
DCM Ethanol Phenol
acid acetate e
Condensed
structural
formula
Physical state at Liquid Liquid Liquid Liquid Solid Liquid Liquid
RT (solid, liquid or
gas)
Appearance Clear Clear Clear Clear Crystalli Clear Clear
(Solid: amorphous, ne
crystals
Liquid: viscous,
watery etc.)
Color Colorless Colorless Colorless Orange White Colorless colorless
Diesel- Kerosen Alcohol- Burnt Odorless Plastic- Ammonia
Odor like odor e-like like odor plastic- like odor -like odor
odor like odor
Solubility in:
(soluble or
insoluble; miscible
and immiscible)
Miscible Immiscib Miscible Slightly Slightly Slightly Miscible
a. Water
le miscible soluble miscible
Slightly Immiscib Miscible Miscible: Soluble Miscible Miscible
b. 5% NaOH miscible le orange
to white
Slightly Immiscib Miscible Miscible: Slightly Miscible Miscible
c. 5% HCl miscible le orange soluble
to white
Reaction with
litmus (acidic,
neutral, basic)
Red to Red to Red to Red to Red to Red to Red to
a. Red red: red: red: red: red: red: blue:
Neutral Neutral Neutral Acidic Acidic Neutral Basic
Blue to Blue to Blue to Blue to Blue to Blue to Blue to
b. Blue blue: blue: blue: Red: red: blue: blue:
Neutral Neutral Neutral Acidic Acidic Neutral Basic
Combustion/Igni Flammabl Non- Flammabl Non- Non- Flammabl Flammabl
tion (Flammable e: yellow- flammab e: blue flammab flammab e: yellow e: blue
or non-flammable) orange le and le le
PHA 615 LABORATORY EXPERIMENT 6 | Page 2 of 4
orange
C-C C-Cl O-H O-H C=C ar. C=O C-H
stretch: stretch: stretch: stretch: Stretch: stretch: stretch:
1200- 580-780, 3700- 3700- 1620- 1740- 3000-
1800, strong 3100, 3100, 1480, 1710, 2840,
very C-C very very medium strong strong
weak stretch: strong strong to weak C-O C-H bend:
C-H 1200- O-H O-H C-H stretch: 1475-
bend: 800, bend: bend: stretch: 1245- 1350,
1435- very 1420- 1420- 3100- 1190 strong to
1350, weak 1340, 1340, 3000, C-H medium
strong to C-H medium medium medium stretch: C-C
medium stretch: to weak to weak to weak 3000- stretch:
C-H 3000- C-O C-O O-H 2840, 1200-
stretch: 2840, stretch: stretch: stretch: strong 800, very
3000- strong 1230- 1230- 3300- C-H weak
2840, 1000, 1000, 2500, bend: N-H
strong strong to strong to broad 1475- stretch:
Description of IR
medium medium strong 1350, 3500-
spectra
C-C C=C ar. O-H strong to 3300 &
stretch: Stretch: bend: medium 3400-
1200- 1620- 1440- C-C 3200,
800. very 1480, 1390, stretch: strong
weak medium medium 1200- N-H
C-H to weak C=O 800, very bend:
stretch: C-C stretch: weak 1640-
3000- stretch: 1730- 1560,
2840, 900-600, 1680, strong &
strong strong to strong 900-650,
C-H medium C-O broad
bend: C-H stretch: medium
1475- stretch: 1320-
1350, 3100- 1210,
strong to 3000, strong
medium medium
to weak
Questions:
1. Based on the table, which of the organic compounds:
a. Is polar Dichloromethane, Ethanol, Phenol, Benzoic acid, Ethyl acetate, Ethylamine
b. Is acidic Phenol, Benzoic acid
c. Is basic Ethylamine
d. Has the strongest IMF Benzoic acid
at RT
2. Which organic compound/s will char on ignition? Explain why this is possible.
PHA 615 LABORATORY EXPERIMENT 6 | Page 3 of 4
Carbohydrates are compounds which will char on ignition because they are made up of carbon. By
heating, charring removes hydrogen and oxygen from the solid. Thus, leaving a char that is primarily
composed of carbon.
References:
Barcellano, H. (2015). Comparative Investigation of Organic Compounds. Retrieved March 2020 from
https://www.scribd.com/document/291548053/Experiment-6-Comparative-Investigation-of-Organic-
Compounds-Formal-Report
Datu, R. (2016). Experiment 6. Retrieved March 2020 onhttps://prezi.com/nmgkeioizr-c/experiment-no-
6/?fbclid=IwAR3IZkozJDPTZUbuGgR5d-nYGNnAqVQeuDFoeQH7cjYT5Sg32qRW7fjhHWU
Formulas of Inorganic and Organic Compounds. (n.d.). ChemWiki. Retrieved March 2020 from
http://chemwiki.ecdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atom
ic_Theory/Chemical_Compounds/Formulas_of_Inorganic_and_Organic_Compounds
National Center for Biotechnology Information. PubChem Database. Benzoic acid, CID=243. Retrieved
March 2020 from https://pubchem.ncbi.nlm.nih.gov/compound/Benzoic-acid
National Center for Biotechnology Information. PubChem Database. Ethylamine, CID=6341. Retrieved
March 2020 from https://pubchem.ncbi.nlm.nih.gov/compound/Ethylamine
PHA 615 LABORATORY EXPERIMENT 6 | Page 4 of 4
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Usp Description and SolubilityDocument1 pageUsp Description and SolubilityvafaashkNo ratings yet
- Print Expt7 Lab ReportDocument7 pagesPrint Expt7 Lab ReportShaliza Hernandez100% (2)
- CottonDocument16 pagesCottonKushagradhi DebnathNo ratings yet
- PHA6111 Lab ACTIVITY 2 HERNANDEZDocument4 pagesPHA6111 Lab ACTIVITY 2 HERNANDEZGrace HernandezNo ratings yet
- Wiki Anal Tit Nonaqueous TitrationDocument5 pagesWiki Anal Tit Nonaqueous TitrationMira JunitaNo ratings yet
- Lab Manual Writeup - Flower Liq-Liq SeparationDocument10 pagesLab Manual Writeup - Flower Liq-Liq SeparationNiharika MalikNo ratings yet
- 5350-5354 Reference Tables - Description and Solubility - ADocument5 pages5350-5354 Reference Tables - Description and Solubility - Apate malabananNo ratings yet
- Descrição e SolubilidadeDocument71 pagesDescrição e Solubilidadereg.analista10No ratings yet
- PHA615 LAB Experiment 4Document3 pagesPHA615 LAB Experiment 4POMPEYO BARROGANo ratings yet
- Chemistry, Water, and Buffers M123Document35 pagesChemistry, Water, and Buffers M123A-Naeem To'mah Al-sawaieNo ratings yet
- Post Lab NotesDocument18 pagesPost Lab Notesshervintancruzado91% (11)
- Lab Manual NÂ3 2023 Acid Base TitrationDocument5 pagesLab Manual NÂ3 2023 Acid Base TitrationabderraouftabaniNo ratings yet
- Acyl CompoundsDocument35 pagesAcyl Compoundsami tiuNo ratings yet
- Chapter 19 Acids and Bases PPT Glembocki 2017Document51 pagesChapter 19 Acids and Bases PPT Glembocki 2017niaNo ratings yet
- 3 - Acid Base Titration in Nonaqueous - DSWDocument27 pages3 - Acid Base Titration in Nonaqueous - DSWbrianNo ratings yet
- USP 42 Description & Relative SolubilityDocument85 pagesUSP 42 Description & Relative Solubilityirhamfauzzi100% (3)
- Jimin - Lesson19.2C (J)Document8 pagesJimin - Lesson19.2C (J)ClaraNo ratings yet
- Non Aqueous TitrationkkkDocument7 pagesNon Aqueous Titrationkkksatheeshpharma6No ratings yet
- Acid-Base Titration in Nonaqueous SolventsDocument6 pagesAcid-Base Titration in Nonaqueous Solventsliz_hobbs79No ratings yet
- Properties of Organic Compounds With Carbonyl GroupDocument14 pagesProperties of Organic Compounds With Carbonyl GroupnadyahginiceNo ratings yet
- Lesson - Acids, Alkalis, and NeutralisationDocument21 pagesLesson - Acids, Alkalis, and NeutralisationĐỗ NguyênNo ratings yet
- Physical Properties of Organic Compounds: Mr. Maywan HarionoDocument27 pagesPhysical Properties of Organic Compounds: Mr. Maywan HarionoJudy MelegritoNo ratings yet
- Acid and Base ReactionsDocument4 pagesAcid and Base ReactionsAntoline Natasha RayappanNo ratings yet
- FTIR Infrared Spectroscopy Absorption TableDocument4 pagesFTIR Infrared Spectroscopy Absorption TablePankaj Kumar PalNo ratings yet
- Org Chem Ost LabDocument21 pagesOrg Chem Ost Labjullian marasiganNo ratings yet
- Neutralization PrelimsDocument3 pagesNeutralization PrelimsJan Paolo TabbalNo ratings yet
- 08 - Infrared Spectroscopy ManualDocument5 pages08 - Infrared Spectroscopy ManualShubham BobadeNo ratings yet
- Dyes & Color by Mansoor IqbalDocument8 pagesDyes & Color by Mansoor Iqbalmansoor iqbal100% (4)
- Acids and Bases: PGCC CHM 101 SinexDocument24 pagesAcids and Bases: PGCC CHM 101 SinexRisna YusniNo ratings yet
- Acids and Bases: PGCC CHM 101 SinexDocument24 pagesAcids and Bases: PGCC CHM 101 Sinex7-SAL 2022No ratings yet
- ف-2-1 2Document18 pagesف-2-1 2Noor FarhanNo ratings yet
- Chem 4Document103 pagesChem 4César Arenas100% (1)
- Non Aqeuous TitrationDocument7 pagesNon Aqeuous Titrationsurabhi tadeNo ratings yet
- BCH 303 Lecture 1Document20 pagesBCH 303 Lecture 1tyhana24No ratings yet
- Solutions 2Document29 pagesSolutions 2Lawrence CarlNo ratings yet
- Acids and BasesDocument5 pagesAcids and BasesLidia CavalieriNo ratings yet
- Acid and Alkali Worksheet 2Document3 pagesAcid and Alkali Worksheet 2Shafiqah Airadz100% (1)
- Acids and Bases: Regents: Chapter 19 P. 586 - 629 Honors: Chapters 20, 21 P. 576 - 643Document103 pagesAcids and Bases: Regents: Chapter 19 P. 586 - 629 Honors: Chapters 20, 21 P. 576 - 643Zaina ZaliraNo ratings yet
- Welcome To My PresentationDocument17 pagesWelcome To My PresentationMrym NbNo ratings yet
- Hsslive Xii Chemistry Lab VOLUMETRIC ANALYSIS TitrationsDocument16 pagesHsslive Xii Chemistry Lab VOLUMETRIC ANALYSIS Titrationsmalavikasunil107No ratings yet
- Acid & Bases 11.1-11.3Document42 pagesAcid & Bases 11.1-11.3harshvaardhanNo ratings yet
- Acid and BaseDocument20 pagesAcid and BaseChris MaNo ratings yet
- Investigatory Project Chemistry PH in Everyday LifeDocument23 pagesInvestigatory Project Chemistry PH in Everyday LifeHarshita Jain67% (3)
- Fedor Kudriavtcev - iGCSE - Chem - Worksheet 16 - Acids, Alkalis & TitrationDocument2 pagesFedor Kudriavtcev - iGCSE - Chem - Worksheet 16 - Acids, Alkalis & Titrationyeet0624kfd0624No ratings yet
- Non Aqueous TitrationDocument31 pagesNon Aqueous TitrationRavi KaushikNo ratings yet
- Acid and BasesDocument27 pagesAcid and BasesdaphneleixiNo ratings yet
- Acids and BasesDocument5 pagesAcids and BasesMuniel Yuri NazarNo ratings yet
- Acid and Alkalis Glossary-PosterDocument1 pageAcid and Alkalis Glossary-PosterEuriz RamosNo ratings yet
- Acids, Bases & BuffersDocument8 pagesAcids, Bases & BuffersChetan JainNo ratings yet
- Water: The Solvent For Biochemical ReactionsDocument53 pagesWater: The Solvent For Biochemical ReactionssarahyahayaNo ratings yet
- Acids, Bases and Salts: Experiment 7Document15 pagesAcids, Bases and Salts: Experiment 7asfass sfasfasfasNo ratings yet
- YT Acid Base and Salt 1Document77 pagesYT Acid Base and Salt 1reyanshNo ratings yet
- Act1 2Section1DRiboDelDocument15 pagesAct1 2Section1DRiboDelRIBO, DELNo ratings yet
- Indicators ExperimentDocument3 pagesIndicators Experimentdhruv.sachdevaNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Pigments, Paint and Painting: A practical book for practical menFrom EverandPigments, Paint and Painting: A practical book for practical menNo ratings yet
- Autonomic Nervous System Drugs: Endogenous AdrenergicsDocument23 pagesAutonomic Nervous System Drugs: Endogenous AdrenergicsGrace HernandezNo ratings yet
- Polyfunctional Prac SetDocument6 pagesPolyfunctional Prac SetGrace Hernandez100% (1)
- Experiment 2: Postlab Qualitative Tests For Proteins RecallDocument15 pagesExperiment 2: Postlab Qualitative Tests For Proteins RecallGrace HernandezNo ratings yet
- HERNANDEZ Assign3 PDFDocument3 pagesHERNANDEZ Assign3 PDFGrace HernandezNo ratings yet
- Phyphar Lab First ShiftingDocument5 pagesPhyphar Lab First ShiftingGrace HernandezNo ratings yet
- Dispensing 1 PrescriptionDocument2 pagesDispensing 1 PrescriptionGrace HernandezNo ratings yet
- Hernandez Theo Summative ExamDocument1 pageHernandez Theo Summative ExamGrace HernandezNo ratings yet
- Hernandez, Grace R. 2A-PH Assignment No. 2Document3 pagesHernandez, Grace R. 2A-PH Assignment No. 2Grace HernandezNo ratings yet
- Hernandez, Grace R. 2A-PH Assignment No. 1Document3 pagesHernandez, Grace R. 2A-PH Assignment No. 1Grace HernandezNo ratings yet
- Ficia L: Erythromycin OintmentDocument2 pagesFicia L: Erythromycin OintmentGrace HernandezNo ratings yet
- (SANTOS) Part 1 Unit 5 FINANCIAL MANAGEMENTDocument34 pages(SANTOS) Part 1 Unit 5 FINANCIAL MANAGEMENTGrace HernandezNo ratings yet
- Rizal Readings 1Document5 pagesRizal Readings 1Grace HernandezNo ratings yet
- Dispensing Lec Activity 1 and 2Document2 pagesDispensing Lec Activity 1 and 2Grace HernandezNo ratings yet
- Micropara Lec Unit 1 LaDocument1 pageMicropara Lec Unit 1 LaGrace HernandezNo ratings yet
- USP NF HydrocortisoneDocument2 pagesUSP NF HydrocortisoneGrace HernandezNo ratings yet
- USP NF NystatinDocument1 pageUSP NF NystatinGrace HernandezNo ratings yet
- INORG LAB Reactions of Alkali GroupDocument1 pageINORG LAB Reactions of Alkali GroupGrace HernandezNo ratings yet
- Virus: Clostridium Tetani - Cause TetanusDocument1 pageVirus: Clostridium Tetani - Cause TetanusGrace HernandezNo ratings yet
- Fundamental Features of Microbiology: VirusesDocument21 pagesFundamental Features of Microbiology: VirusesGrace HernandezNo ratings yet
- Case Application For Decision-Making PDFDocument2 pagesCase Application For Decision-Making PDFGrace HernandezNo ratings yet
- GRH - 1aph - 1Document9 pagesGRH - 1aph - 1Grace HernandezNo ratings yet
- Performance Task in Physics: University of Santo TomasDocument4 pagesPerformance Task in Physics: University of Santo TomasGrace HernandezNo ratings yet
- Participatory Capacities and Vulnerabilities Assessment Part 1Document5 pagesParticipatory Capacities and Vulnerabilities Assessment Part 1Grace HernandezNo ratings yet
- Course Plan 2019 - Pha 617 (Pharmacy Administration, Leadership, and Management)Document14 pagesCourse Plan 2019 - Pha 617 (Pharmacy Administration, Leadership, and Management)Grace Hernandez100% (1)
- Saint Agnes Parish: Evangelization and Service To OthersDocument3 pagesSaint Agnes Parish: Evangelization and Service To OthersGrace HernandezNo ratings yet
- Pha619 Lec Chap 2Document9 pagesPha619 Lec Chap 2Grace HernandezNo ratings yet
- TissueDocument1 pageTissueGrace HernandezNo ratings yet
- PHA618 - Anatomical TermsDocument4 pagesPHA618 - Anatomical TermsGrace Hernandez100% (1)
- Chem 31.1 Postlab 9Document1 pageChem 31.1 Postlab 9Sellina SyNo ratings yet
- List of Hazardous Chemical As Per Notification Dated 19 TH January 2000Document18 pagesList of Hazardous Chemical As Per Notification Dated 19 TH January 2000saikumar selaNo ratings yet
- Aluminium NitrateDocument3 pagesAluminium NitrategumtammNo ratings yet
- RA Sir JEE PYQ Organic ChemistryDocument191 pagesRA Sir JEE PYQ Organic Chemistrydash gupta100% (4)
- Organic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusDocument6 pagesOrganic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusMadison FullerNo ratings yet
- Chemical Composition of PetroleumDocument18 pagesChemical Composition of PetroleumVeera Reddy BijjaramNo ratings yet
- SALTINGDocument6 pagesSALTINGGret ChenNo ratings yet
- Follow Up Sheet GUDocument26 pagesFollow Up Sheet GUnomanNo ratings yet
- C Family Silicon, Silicates and Their TypesDocument6 pagesC Family Silicon, Silicates and Their TypesUsman GhaniNo ratings yet
- Ammonium GlycyrrhizateDocument2 pagesAmmonium GlycyrrhizateAndré C. de PaulaNo ratings yet
- Physical Pharmacy - Second Stage Lecture 9Document82 pagesPhysical Pharmacy - Second Stage Lecture 9atheeralmadridi48No ratings yet
- Sintesis de IndolesDocument14 pagesSintesis de IndolesLili AmadorNo ratings yet
- MATHEMATICS - (12th & 13th) Paper-2Document3 pagesMATHEMATICS - (12th & 13th) Paper-2Raju SinghNo ratings yet
- June 2020 (R) QPDocument28 pagesJune 2020 (R) QPafzalafsaNo ratings yet
- Food Chemistry Volume 128 Issue 3 2011 (Doi 10.1016 - J.foodchem.2011.03.075) He-Ya Wang He Qian Wei-Rong Yao - Melanoidins P PDFDocument12 pagesFood Chemistry Volume 128 Issue 3 2011 (Doi 10.1016 - J.foodchem.2011.03.075) He-Ya Wang He Qian Wei-Rong Yao - Melanoidins P PDFOktavia Rahayu ANo ratings yet
- Reaction Mechanism Worksheet PDFDocument60 pagesReaction Mechanism Worksheet PDFSacchitDSheth67% (3)
- Chemistry Revision Worksheet CH1Document16 pagesChemistry Revision Worksheet CH1gcubeyyNo ratings yet
- Prediksi NamaDocument44 pagesPrediksi NamaNurlailatush SholihahNo ratings yet
- Class 12 CBSE Electrochemistry QuestionsDocument1 pageClass 12 CBSE Electrochemistry Questionsankitsingh90No ratings yet
- Titration EssentialsDocument87 pagesTitration EssentialsFabio GreenNo ratings yet
- EdExcel A Level Chemistry Unit 7 Mark Scheme Jan 2000Document4 pagesEdExcel A Level Chemistry Unit 7 Mark Scheme Jan 2000MashiatUddinNo ratings yet
- Eng Chem Lecture NotesDocument2 pagesEng Chem Lecture NotesJunell TadinaNo ratings yet
- Aqa Chemistry Jan 2011 Past Paper PDFDocument16 pagesAqa Chemistry Jan 2011 Past Paper PDFTiffany MaddoxNo ratings yet
- Trouble Shooting in Dyeing ProcessDocument26 pagesTrouble Shooting in Dyeing ProcessAnonymous wA6NGuyklDNo ratings yet
- Shell HD Premium Coolant N Pre Diluted 50 - 50Document1 pageShell HD Premium Coolant N Pre Diluted 50 - 50Binar KusumahNo ratings yet
- Chem ReportDocument10 pagesChem Reportzyan himorNo ratings yet
- Chem 1Document111 pagesChem 1C1A 05 Ashwina JNo ratings yet
- List ASTM For Water Testing StandardsDocument13 pagesList ASTM For Water Testing StandardsAnonymous a19X9GHZNo ratings yet
- Etherificacion Del GlicerolDocument8 pagesEtherificacion Del GlicerolGastón Miranda GarcíaNo ratings yet
- Hemsheela Model School Durgapur Term-Ii Examination-2022 Chemistry Marking Scheme Class-XiDocument6 pagesHemsheela Model School Durgapur Term-Ii Examination-2022 Chemistry Marking Scheme Class-XiBaichitra MondalNo ratings yet