Professional Documents
Culture Documents
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
Uploaded by
Ankush BiswasCopyright:
Available Formats
You might also like
- Test Bank For Biochemistry The Molecular Basis of Life 7th Edition James R Mckee Trudy MckeeDocument19 pagesTest Bank For Biochemistry The Molecular Basis of Life 7th Edition James R Mckee Trudy MckeeVince Sjogren100% (39)
- A Text Book of Human Anatomy & Physiology For Pharm.D I YearDocument3 pagesA Text Book of Human Anatomy & Physiology For Pharm.D I YearAnkush BiswasNo ratings yet
- Presentation - Jeddah Airport 2 ISTP (KOM)Document44 pagesPresentation - Jeddah Airport 2 ISTP (KOM)Mana DiaaNo ratings yet
- Material Safety Data Sheet: Oxbow Calcining LLCDocument4 pagesMaterial Safety Data Sheet: Oxbow Calcining LLCFinka Pertama PutriNo ratings yet
- UNIT III GastrointestinalagentsAntacidDocument9 pagesUNIT III GastrointestinalagentsAntacidgalihNo ratings yet
- Gastrointestinal AgentsDocument28 pagesGastrointestinal AgentsAbdur RaquibNo ratings yet
- UNIT III GastrointestinalagentsAntacidDocument9 pagesUNIT III GastrointestinalagentsAntacidnamrataNo ratings yet
- PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)Document9 pagesPHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)MD REFATNo ratings yet
- CHEMISTRY Project For Class 12Document19 pagesCHEMISTRY Project For Class 12Naveen Doss100% (1)
- Chemistry Project To To Determine Which Antacid Neutralizes Stomach Acid Most To Determine Which Antacid Could Neutralize The Most Stomach AcidDocument8 pagesChemistry Project To To Determine Which Antacid Neutralizes Stomach Acid Most To Determine Which Antacid Could Neutralize The Most Stomach AcidAnkur GuptaNo ratings yet
- Action of Antacids: Chemistry Investiga Tory ProjectDocument16 pagesAction of Antacids: Chemistry Investiga Tory ProjectSairamRamakrishnanNo ratings yet
- Chapter-16 Chemistry in Everyday LifeDocument9 pagesChapter-16 Chemistry in Everyday Lifeshodhan shettyNo ratings yet
- Chemistry Project ANTACIDDocument17 pagesChemistry Project ANTACIDMAX MusicNo ratings yet
- P'Chem-I Cathartics & InhalantsDocument2 pagesP'Chem-I Cathartics & InhalantsGhubaya CopNo ratings yet
- P'Chem-I Cathartics & Inhalants PDFDocument2 pagesP'Chem-I Cathartics & Inhalants PDFGhubaya CopNo ratings yet
- Final U-III Content GI TractDocument39 pagesFinal U-III Content GI Tractapple crazypineappleNo ratings yet
- To Determine Which Antacid Could Neutralize The Most Stomach AcidDocument14 pagesTo Determine Which Antacid Could Neutralize The Most Stomach Acidanmol sandhuNo ratings yet
- Chapt. V Gastrointestinal Agents: Inorganic Agents Used To Treat Gastro-Intestinal Tract DisordersDocument9 pagesChapt. V Gastrointestinal Agents: Inorganic Agents Used To Treat Gastro-Intestinal Tract DisordersJoy VergaraNo ratings yet
- Unit 2 Antacids Aluminium Hydroxide Gel Magnesium Hydroxide MagaldrateDocument6 pagesUnit 2 Antacids Aluminium Hydroxide Gel Magnesium Hydroxide Magaldrateabhay sharmaNo ratings yet
- GIT Agents 2021Document28 pagesGIT Agents 2021madhanraj7996No ratings yet
- Advanced Academy: Comparitive Study of Commercial AntacidsDocument15 pagesAdvanced Academy: Comparitive Study of Commercial AntacidsGaurav Gupta33% (3)
- Chemstry Invetigatory ProjectDocument24 pagesChemstry Invetigatory ProjectAnsia MeenazNo ratings yet
- Chemistry ProjectDocument17 pagesChemistry Projectsuhana hussain50% (2)
- Experiment No. 12: Preparation of Precipitated Sulfur USPDocument7 pagesExperiment No. 12: Preparation of Precipitated Sulfur USPAlessandra Joy M. RoceroNo ratings yet
- Gastrointestinal Agents: Ana Marie L. Rubenicia, RPHDocument31 pagesGastrointestinal Agents: Ana Marie L. Rubenicia, RPHEmman AguilarNo ratings yet
- Yashita Agarwala CHEM ProjectDocument22 pagesYashita Agarwala CHEM ProjectYash ParkhiNo ratings yet
- Determination of Acid Neutralizing Capacity of Various Marketed Antacids in NepalDocument34 pagesDetermination of Acid Neutralizing Capacity of Various Marketed Antacids in NepalTshiva Aryal100% (1)
- Study of Acid Neutralizing Capacity Ofvarious Antacid Formulations PDFDocument8 pagesStudy of Acid Neutralizing Capacity Ofvarious Antacid Formulations PDFMuhammad IqbalNo ratings yet
- Chemistry Investigatory AntacidsDocument25 pagesChemistry Investigatory AntacidsJayasree MNo ratings yet
- Topic-: Chemistry Investigatory ProjectDocument18 pagesTopic-: Chemistry Investigatory ProjectRomil TalaichNo ratings yet
- Project On Antacids Cbse ChemDocument15 pagesProject On Antacids Cbse ChemSairamRamakrishnan33% (3)
- Chemistry ProjectDocument16 pagesChemistry Projectarchiekundu15No ratings yet
- Phchem 1a - Activity #2 - Pharmaceutical Aids and Necessities (Summer 2022)Document3 pagesPhchem 1a - Activity #2 - Pharmaceutical Aids and Necessities (Summer 2022)Shopifyy ClothingNo ratings yet
- Analysis of SalivaDocument14 pagesAnalysis of Salivajgbalanay8492antNo ratings yet
- 12a AntacidsDocument17 pages12a AntacidsinshirahNo ratings yet
- Ratan Chem-1Document16 pagesRatan Chem-1sid.dicaprioNo ratings yet
- Chemistry Ivestigatory Project.Document11 pagesChemistry Ivestigatory Project.B.srihariNo ratings yet
- AntacidsDocument16 pagesAntacidsPedroCordovageNo ratings yet
- Food BiochemistryDocument10 pagesFood BiochemistrySaima RiazNo ratings yet
- BpharDocument67 pagesBpharSavitri HegdeNo ratings yet
- Pharmaceutical Lecture 1st Year CollegeDocument8 pagesPharmaceutical Lecture 1st Year Collegebilgeposer123No ratings yet
- Chem ProjectDocument17 pagesChem Projects66hh6ggc2No ratings yet
- Chemistry Project For School Students byDocument13 pagesChemistry Project For School Students byvsetanmayNo ratings yet
- ACTIVITY 5 Pchem 1Document3 pagesACTIVITY 5 Pchem 1Shopifyy ClothingNo ratings yet
- Comparitive Study of Commercial AntacidsDocument41 pagesComparitive Study of Commercial Antacidslulu puluNo ratings yet
- Pharmaceutical Aids and NecessitiesDocument25 pagesPharmaceutical Aids and NecessitiesKyle Angelic DavasolNo ratings yet
- Investigatory ProjectDocument30 pagesInvestigatory ProjectvedanggamezNo ratings yet
- Topical, Gastrointestinal, NI, Radio-, MiscDocument113 pagesTopical, Gastrointestinal, NI, Radio-, MiscArk Olfato ParojinogNo ratings yet
- Delhi Scottish School: Deepak ChandalaDocument24 pagesDelhi Scottish School: Deepak Chandalapyiush jainNo ratings yet
- Antacids Class 12Document7 pagesAntacids Class 12rav_rkdNo ratings yet
- Managing Phosphorus in Agriculture Consulting by SlidesgoDocument11 pagesManaging Phosphorus in Agriculture Consulting by SlidesgopblasyaNo ratings yet
- 3 DglassesDocument6 pages3 DglassesSai SreedharNo ratings yet
- AntacidDocument16 pagesAntacidVikramNo ratings yet
- Chem Project Class 12 With Investigatory Project On ' Antacids'Document15 pagesChem Project Class 12 With Investigatory Project On ' Antacids'SHAHBAN55550% (1)
- New Microsoft Word DocumentDocument14 pagesNew Microsoft Word DocumentApurav KhobragadeNo ratings yet
- Ankit Kumar 1Document14 pagesAnkit Kumar 1Hitesh 1No ratings yet
- LEC 11 Official Inorganic CompoundsDocument28 pagesLEC 11 Official Inorganic CompoundsishafatimapakistaniNo ratings yet
- Antacid - PonbaraniDocument11 pagesAntacid - PonbaranifmdazharNo ratings yet
- HYP E R ACI D I T Y Cause FOR Intake OF AntacidsDocument14 pagesHYP E R ACI D I T Y Cause FOR Intake OF AntacidsamuNo ratings yet
- Chemistry Class 12 Project AntacidsDocument13 pagesChemistry Class 12 Project AntacidsTanishka SinghNo ratings yet
- Chemistry ProjectDocument29 pagesChemistry ProjectDibyadisha ParidaNo ratings yet
- Acidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)Document29 pagesAcidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)jibachha sahNo ratings yet
- Gastrointestinal AgentsDocument72 pagesGastrointestinal Agentsfarooq shah shabbirNo ratings yet
- Factors_Influencing_College_Students_Internship_Selection_DecisionsDocument14 pagesFactors_Influencing_College_Students_Internship_Selection_DecisionsAnkush BiswasNo ratings yet
- CologyDemoNotesDocument50 pagesCologyDemoNotesAnkush BiswasNo ratings yet
- A Text Book of Physical Pharmaceutics-I: September 2017Document3 pagesA Text Book of Physical Pharmaceutics-I: September 2017Ankush BiswasNo ratings yet
- Pharmaminds Sankalp 2026Document29 pagesPharmaminds Sankalp 2026Ankush BiswasNo ratings yet
- Bac AmpicillinDocument2 pagesBac AmpicillinAnkush BiswasNo ratings yet
- Books: ReviewsDocument1 pageBooks: ReviewsAnkush BiswasNo ratings yet
- Organic Chemistry PDFDocument4 pagesOrganic Chemistry PDFAnkush BiswasNo ratings yet
- Proton Pump InhibitorDocument9 pagesProton Pump InhibitorAnkush BiswasNo ratings yet
- Experiment Preparation of Media: 7.0 ObjectivesDocument11 pagesExperiment Preparation of Media: 7.0 ObjectivesAnkush BiswasNo ratings yet
- MetalaxylM 240502 153746Document72 pagesMetalaxylM 240502 153746HidayatRustdyNo ratings yet
- Stichlmair, J. (2010) - Distillation, 3. Processes. Ullmann's Encyclopedia of Industrial Chemistry.Document18 pagesStichlmair, J. (2010) - Distillation, 3. Processes. Ullmann's Encyclopedia of Industrial Chemistry.Silvio Latini SpahnNo ratings yet
- Docsity Laboratory Report About Water and Its PropertiesDocument8 pagesDocsity Laboratory Report About Water and Its PropertiesRAFAELLA SALVE MARIE GAETOSNo ratings yet
- Business Math Final ReportDocument99 pagesBusiness Math Final ReportAbubakar MehmoodNo ratings yet
- Daftar PustakaDocument5 pagesDaftar Pustakaronald andreaNo ratings yet
- 1 - A Study Concerning Intercritical HAZ Microstructure and Toughness in ...Document10 pages1 - A Study Concerning Intercritical HAZ Microstructure and Toughness in ...Filipe RenanNo ratings yet
- Abstract of Final Bill OIL, PS-10 BarauniDocument21 pagesAbstract of Final Bill OIL, PS-10 BarauniMd Mukarram RezaNo ratings yet
- PRS - Smart Tap - Hot Tap FittingDocument2 pagesPRS - Smart Tap - Hot Tap FittingMario Rivera AlonsoNo ratings yet
- Cable HMWPE - Proteccion CatodicaDocument2 pagesCable HMWPE - Proteccion CatodicaEdwin Alfaro GNo ratings yet
- Engineering Guide User InterfacesDocument12 pagesEngineering Guide User Interfacessav33No ratings yet
- Development in Wastewater Treatment Research and Processes: Microbial Degradation of Xenobiotics Through Bacterial and Fungal Approach Maulin P. ShahDocument52 pagesDevelopment in Wastewater Treatment Research and Processes: Microbial Degradation of Xenobiotics Through Bacterial and Fungal Approach Maulin P. Shahchristy.delano512100% (6)
- Traditional Butter and Ghee Production, Processing and Handling in Ethiopia: A ReviewDocument11 pagesTraditional Butter and Ghee Production, Processing and Handling in Ethiopia: A ReviewAlexandra CîrsteaNo ratings yet
- Tabela Formulacoes FertilizantesDocument1 pageTabela Formulacoes FertilizantesGeraldo Aparecido SantosNo ratings yet
- Nanomedicine and Cancer: by Nicole Chia Poh HuiDocument9 pagesNanomedicine and Cancer: by Nicole Chia Poh HuiLas Cicas KchibiusaNo ratings yet
- Utilization of Palm Oil Mill Effluent - Avoiding CH Emission PT Agricinal, Bengkulu - IndonesiaDocument10 pagesUtilization of Palm Oil Mill Effluent - Avoiding CH Emission PT Agricinal, Bengkulu - Indonesiafebian_sam22No ratings yet
- Health Benefits and Possible Risks of Herbal Medicine: March 2016Document21 pagesHealth Benefits and Possible Risks of Herbal Medicine: March 2016Juan GomezNo ratings yet
- Pro Elite Analyzer Operation Manual 4001051 Rev A PDFDocument36 pagesPro Elite Analyzer Operation Manual 4001051 Rev A PDFintermountainwaterNo ratings yet
- S6900QLDocument4 pagesS6900QLmechkashanNo ratings yet
- Brochure - M Series en - Pompa OBLDocument6 pagesBrochure - M Series en - Pompa OBLAmrina RosyadaNo ratings yet
- "Multi Village Waste Management System Huligi": Waste Dumping Yard and Heaping of WasteDocument3 pages"Multi Village Waste Management System Huligi": Waste Dumping Yard and Heaping of WasteravibasarihalliNo ratings yet
- Japan Engine CatalgoDocument40 pagesJapan Engine CatalgoKarim Sowley DelgadoNo ratings yet
- Comparative Investigation On Tensile Performance of FRP Bars After Exposure To Water, Seawater, and Alkaline SolutionsDocument12 pagesComparative Investigation On Tensile Performance of FRP Bars After Exposure To Water, Seawater, and Alkaline Solutionssherif fodaNo ratings yet
- Fluconazole - Customer - Notification - Recall Communication - LetterDocument1 pageFluconazole - Customer - Notification - Recall Communication - Lettermohammed shaffi abdul rahmanNo ratings yet
- Balston Nitrogen GeneratorDocument10 pagesBalston Nitrogen GeneratorNguyễn NgọcNo ratings yet
- 3500-Mn Manganese (Editorial Revisions, 2011)Document3 pages3500-Mn Manganese (Editorial Revisions, 2011)Gustavo Baccho Jorge FilhoNo ratings yet
- Paints, CoatingsDocument9 pagesPaints, CoatingsazozinlcNo ratings yet
- Capillary Tube For Refrigeration and Air Conditioning SystemsDocument3 pagesCapillary Tube For Refrigeration and Air Conditioning SystemsIjazzzAliNo ratings yet
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
Uploaded by
Ankush BiswasOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Acidifiers)
Uploaded by
Ankush BiswasCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/321938509
PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal agents
(Acidifiers)
Presentation · December 2017
DOI: 10.13140/RG.2.2.28629.93926
CITATIONS READS
0 12,961
1 author:
Dr Sumanta Mondal
GITAM (Deemed to be University)
206 PUBLICATIONS 294 CITATIONS
SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Research and Development of Nanoparticle Characterization Methods Research and Development of Nanoparticle Characterization Methods View project
Methods Development and Validation of Pharmaceutical Dosage Forms View project
All content following this page was uploaded by Dr Sumanta Mondal on 20 December 2017.
The user has requested enhancement of the downloaded file.
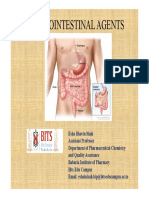
PHARMACEUTICAL INORGANIC CHEMISTRY (BP104T) UNIT – III: Gastrointestinal agents (Acidifiers)
- Acidifiers are inorganic chemicals that either produce or become acid.
- These chemicals increase the level of gastric acid in the stomach when ingested, thus decreasing the stomach pH.
- These are many types of acidifiers but the main four types are:
i. Gastric acidifiers, used in controlling pH in stomach.
ii. Urinary acidifiers, used in controlling pH in urine.
iii. Systemic acidifiers, used in controlling pH in all the parts of body.
- ACHLORHYDRIA: In patients suffering from achlorhydria, there is deficient secretion of HCl in stomach. In such
cases acidifiers are useful in providing the necessary acidity for the proper digestion of food. Systemic acidifiers are
those which, when given usually by injection, act by reducing the alkali reserve in the body and are also useful in
reducing metabolic alkaloids.
Ammonium chloride
Molecular formula NH4Cl
Molar mass 53.49 g/mol
Synonym Sal ammoniac
Solutions of ammonium chloride are mildly acidic.
Physical Properties
Appearance: White solid, hygroscopic Odor: Odourless Taste: Cooling saline Density: 1.5274 g/cm3
Melting point: 3380 C (decomposes, sublimes) Solubility: free soluble in water and glycerol, Sparingly soluble in alcohol
Preparation:
- Ammonium chloride prepared through the Solvay process:
CO2 + 2 NH3 + 2 NaCl + H2O → 2 NH4Cl + Na2CO3
- Ammonium chloride is prepared commercially by combining ammonia (NH3) with either hydrogen chloride (gas) or
hydrochloric acid (water solution): NH3 + HCl → NH4Cl
Reactions
- Ammonium chloride appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride
gas: NH4Cl → NH3 + HCl
- Ammonium chloride reacts with a strong base, like sodium hydroxide, to release ammonia gas:
NH4Cl + NaOH → NH3 + NaCl + H2O
- Similarly, ammonium chloride also reacts with alkali metal carbonates at elevated temperatures, giving ammonia and
alkali metal chloride: 2 NH4Cl + Na2CO3 → 2 NaCl + CO2 + H2O + 2 NH3
Assay
- Dissolve 1.000 g of Ammonium chloride in 20 ml of water and add a mixture of 5 ml of formaldehyde solution, with
few drops of phenolphthalein solution. After 1 min to 2 min, titrate slowly with 1M sodium hydroxide.
- 1 ml of 1M sodium hydroxide is equivalent to 53.49 mg of NH4Cl.
Uses:
- Ammonium chloride is used as an expectorant in cough medicine. Its expectorant action is caused by irritative action
on the bronchial mucosa, which causes the production of excess respiratory tract fluid, which presumably is easier to
cough up.
- Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting.
- Ammonium chloride is used as a systemic acidifying agent in treatment of severe metabolic alkalosis.
- The main application of ammonium chloride is as a nitrogen source in fertilizers.
- Ammonium chloride is used as a flux in preparing metals to be tin coated, galvanized or soldered.
Dose: 1 to 2 gm (As systemic acidifier); 0.3 to 0.5 gm (Expectorant)
Storage: Store in highly closed container.
Dr. Sumanta Mondal_ Lecturer Notes_B.Pharm-I Sem._GITAM UNIVERSITY 1
E-mail: logonchemistry@gmail.com
View publication stats
You might also like
- Test Bank For Biochemistry The Molecular Basis of Life 7th Edition James R Mckee Trudy MckeeDocument19 pagesTest Bank For Biochemistry The Molecular Basis of Life 7th Edition James R Mckee Trudy MckeeVince Sjogren100% (39)
- A Text Book of Human Anatomy & Physiology For Pharm.D I YearDocument3 pagesA Text Book of Human Anatomy & Physiology For Pharm.D I YearAnkush BiswasNo ratings yet
- Presentation - Jeddah Airport 2 ISTP (KOM)Document44 pagesPresentation - Jeddah Airport 2 ISTP (KOM)Mana DiaaNo ratings yet
- Material Safety Data Sheet: Oxbow Calcining LLCDocument4 pagesMaterial Safety Data Sheet: Oxbow Calcining LLCFinka Pertama PutriNo ratings yet
- UNIT III GastrointestinalagentsAntacidDocument9 pagesUNIT III GastrointestinalagentsAntacidgalihNo ratings yet
- Gastrointestinal AgentsDocument28 pagesGastrointestinal AgentsAbdur RaquibNo ratings yet
- UNIT III GastrointestinalagentsAntacidDocument9 pagesUNIT III GastrointestinalagentsAntacidnamrataNo ratings yet
- PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)Document9 pagesPHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)MD REFATNo ratings yet
- CHEMISTRY Project For Class 12Document19 pagesCHEMISTRY Project For Class 12Naveen Doss100% (1)
- Chemistry Project To To Determine Which Antacid Neutralizes Stomach Acid Most To Determine Which Antacid Could Neutralize The Most Stomach AcidDocument8 pagesChemistry Project To To Determine Which Antacid Neutralizes Stomach Acid Most To Determine Which Antacid Could Neutralize The Most Stomach AcidAnkur GuptaNo ratings yet
- Action of Antacids: Chemistry Investiga Tory ProjectDocument16 pagesAction of Antacids: Chemistry Investiga Tory ProjectSairamRamakrishnanNo ratings yet
- Chapter-16 Chemistry in Everyday LifeDocument9 pagesChapter-16 Chemistry in Everyday Lifeshodhan shettyNo ratings yet
- Chemistry Project ANTACIDDocument17 pagesChemistry Project ANTACIDMAX MusicNo ratings yet
- P'Chem-I Cathartics & InhalantsDocument2 pagesP'Chem-I Cathartics & InhalantsGhubaya CopNo ratings yet
- P'Chem-I Cathartics & Inhalants PDFDocument2 pagesP'Chem-I Cathartics & Inhalants PDFGhubaya CopNo ratings yet
- Final U-III Content GI TractDocument39 pagesFinal U-III Content GI Tractapple crazypineappleNo ratings yet
- To Determine Which Antacid Could Neutralize The Most Stomach AcidDocument14 pagesTo Determine Which Antacid Could Neutralize The Most Stomach Acidanmol sandhuNo ratings yet
- Chapt. V Gastrointestinal Agents: Inorganic Agents Used To Treat Gastro-Intestinal Tract DisordersDocument9 pagesChapt. V Gastrointestinal Agents: Inorganic Agents Used To Treat Gastro-Intestinal Tract DisordersJoy VergaraNo ratings yet
- Unit 2 Antacids Aluminium Hydroxide Gel Magnesium Hydroxide MagaldrateDocument6 pagesUnit 2 Antacids Aluminium Hydroxide Gel Magnesium Hydroxide Magaldrateabhay sharmaNo ratings yet
- GIT Agents 2021Document28 pagesGIT Agents 2021madhanraj7996No ratings yet
- Advanced Academy: Comparitive Study of Commercial AntacidsDocument15 pagesAdvanced Academy: Comparitive Study of Commercial AntacidsGaurav Gupta33% (3)
- Chemstry Invetigatory ProjectDocument24 pagesChemstry Invetigatory ProjectAnsia MeenazNo ratings yet
- Chemistry ProjectDocument17 pagesChemistry Projectsuhana hussain50% (2)
- Experiment No. 12: Preparation of Precipitated Sulfur USPDocument7 pagesExperiment No. 12: Preparation of Precipitated Sulfur USPAlessandra Joy M. RoceroNo ratings yet
- Gastrointestinal Agents: Ana Marie L. Rubenicia, RPHDocument31 pagesGastrointestinal Agents: Ana Marie L. Rubenicia, RPHEmman AguilarNo ratings yet
- Yashita Agarwala CHEM ProjectDocument22 pagesYashita Agarwala CHEM ProjectYash ParkhiNo ratings yet
- Determination of Acid Neutralizing Capacity of Various Marketed Antacids in NepalDocument34 pagesDetermination of Acid Neutralizing Capacity of Various Marketed Antacids in NepalTshiva Aryal100% (1)
- Study of Acid Neutralizing Capacity Ofvarious Antacid Formulations PDFDocument8 pagesStudy of Acid Neutralizing Capacity Ofvarious Antacid Formulations PDFMuhammad IqbalNo ratings yet
- Chemistry Investigatory AntacidsDocument25 pagesChemistry Investigatory AntacidsJayasree MNo ratings yet
- Topic-: Chemistry Investigatory ProjectDocument18 pagesTopic-: Chemistry Investigatory ProjectRomil TalaichNo ratings yet
- Project On Antacids Cbse ChemDocument15 pagesProject On Antacids Cbse ChemSairamRamakrishnan33% (3)
- Chemistry ProjectDocument16 pagesChemistry Projectarchiekundu15No ratings yet
- Phchem 1a - Activity #2 - Pharmaceutical Aids and Necessities (Summer 2022)Document3 pagesPhchem 1a - Activity #2 - Pharmaceutical Aids and Necessities (Summer 2022)Shopifyy ClothingNo ratings yet
- Analysis of SalivaDocument14 pagesAnalysis of Salivajgbalanay8492antNo ratings yet
- 12a AntacidsDocument17 pages12a AntacidsinshirahNo ratings yet
- Ratan Chem-1Document16 pagesRatan Chem-1sid.dicaprioNo ratings yet
- Chemistry Ivestigatory Project.Document11 pagesChemistry Ivestigatory Project.B.srihariNo ratings yet
- AntacidsDocument16 pagesAntacidsPedroCordovageNo ratings yet
- Food BiochemistryDocument10 pagesFood BiochemistrySaima RiazNo ratings yet
- BpharDocument67 pagesBpharSavitri HegdeNo ratings yet
- Pharmaceutical Lecture 1st Year CollegeDocument8 pagesPharmaceutical Lecture 1st Year Collegebilgeposer123No ratings yet
- Chem ProjectDocument17 pagesChem Projects66hh6ggc2No ratings yet
- Chemistry Project For School Students byDocument13 pagesChemistry Project For School Students byvsetanmayNo ratings yet
- ACTIVITY 5 Pchem 1Document3 pagesACTIVITY 5 Pchem 1Shopifyy ClothingNo ratings yet
- Comparitive Study of Commercial AntacidsDocument41 pagesComparitive Study of Commercial Antacidslulu puluNo ratings yet
- Pharmaceutical Aids and NecessitiesDocument25 pagesPharmaceutical Aids and NecessitiesKyle Angelic DavasolNo ratings yet
- Investigatory ProjectDocument30 pagesInvestigatory ProjectvedanggamezNo ratings yet
- Topical, Gastrointestinal, NI, Radio-, MiscDocument113 pagesTopical, Gastrointestinal, NI, Radio-, MiscArk Olfato ParojinogNo ratings yet
- Delhi Scottish School: Deepak ChandalaDocument24 pagesDelhi Scottish School: Deepak Chandalapyiush jainNo ratings yet
- Antacids Class 12Document7 pagesAntacids Class 12rav_rkdNo ratings yet
- Managing Phosphorus in Agriculture Consulting by SlidesgoDocument11 pagesManaging Phosphorus in Agriculture Consulting by SlidesgopblasyaNo ratings yet
- 3 DglassesDocument6 pages3 DglassesSai SreedharNo ratings yet
- AntacidDocument16 pagesAntacidVikramNo ratings yet
- Chem Project Class 12 With Investigatory Project On ' Antacids'Document15 pagesChem Project Class 12 With Investigatory Project On ' Antacids'SHAHBAN55550% (1)
- New Microsoft Word DocumentDocument14 pagesNew Microsoft Word DocumentApurav KhobragadeNo ratings yet
- Ankit Kumar 1Document14 pagesAnkit Kumar 1Hitesh 1No ratings yet
- LEC 11 Official Inorganic CompoundsDocument28 pagesLEC 11 Official Inorganic CompoundsishafatimapakistaniNo ratings yet
- Antacid - PonbaraniDocument11 pagesAntacid - PonbaranifmdazharNo ratings yet
- HYP E R ACI D I T Y Cause FOR Intake OF AntacidsDocument14 pagesHYP E R ACI D I T Y Cause FOR Intake OF AntacidsamuNo ratings yet
- Chemistry Class 12 Project AntacidsDocument13 pagesChemistry Class 12 Project AntacidsTanishka SinghNo ratings yet
- Chemistry ProjectDocument29 pagesChemistry ProjectDibyadisha ParidaNo ratings yet
- Acidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)Document29 pagesAcidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)jibachha sahNo ratings yet
- Gastrointestinal AgentsDocument72 pagesGastrointestinal Agentsfarooq shah shabbirNo ratings yet
- Factors_Influencing_College_Students_Internship_Selection_DecisionsDocument14 pagesFactors_Influencing_College_Students_Internship_Selection_DecisionsAnkush BiswasNo ratings yet
- CologyDemoNotesDocument50 pagesCologyDemoNotesAnkush BiswasNo ratings yet
- A Text Book of Physical Pharmaceutics-I: September 2017Document3 pagesA Text Book of Physical Pharmaceutics-I: September 2017Ankush BiswasNo ratings yet
- Pharmaminds Sankalp 2026Document29 pagesPharmaminds Sankalp 2026Ankush BiswasNo ratings yet
- Bac AmpicillinDocument2 pagesBac AmpicillinAnkush BiswasNo ratings yet
- Books: ReviewsDocument1 pageBooks: ReviewsAnkush BiswasNo ratings yet
- Organic Chemistry PDFDocument4 pagesOrganic Chemistry PDFAnkush BiswasNo ratings yet
- Proton Pump InhibitorDocument9 pagesProton Pump InhibitorAnkush BiswasNo ratings yet
- Experiment Preparation of Media: 7.0 ObjectivesDocument11 pagesExperiment Preparation of Media: 7.0 ObjectivesAnkush BiswasNo ratings yet
- MetalaxylM 240502 153746Document72 pagesMetalaxylM 240502 153746HidayatRustdyNo ratings yet
- Stichlmair, J. (2010) - Distillation, 3. Processes. Ullmann's Encyclopedia of Industrial Chemistry.Document18 pagesStichlmair, J. (2010) - Distillation, 3. Processes. Ullmann's Encyclopedia of Industrial Chemistry.Silvio Latini SpahnNo ratings yet
- Docsity Laboratory Report About Water and Its PropertiesDocument8 pagesDocsity Laboratory Report About Water and Its PropertiesRAFAELLA SALVE MARIE GAETOSNo ratings yet
- Business Math Final ReportDocument99 pagesBusiness Math Final ReportAbubakar MehmoodNo ratings yet
- Daftar PustakaDocument5 pagesDaftar Pustakaronald andreaNo ratings yet
- 1 - A Study Concerning Intercritical HAZ Microstructure and Toughness in ...Document10 pages1 - A Study Concerning Intercritical HAZ Microstructure and Toughness in ...Filipe RenanNo ratings yet
- Abstract of Final Bill OIL, PS-10 BarauniDocument21 pagesAbstract of Final Bill OIL, PS-10 BarauniMd Mukarram RezaNo ratings yet
- PRS - Smart Tap - Hot Tap FittingDocument2 pagesPRS - Smart Tap - Hot Tap FittingMario Rivera AlonsoNo ratings yet
- Cable HMWPE - Proteccion CatodicaDocument2 pagesCable HMWPE - Proteccion CatodicaEdwin Alfaro GNo ratings yet
- Engineering Guide User InterfacesDocument12 pagesEngineering Guide User Interfacessav33No ratings yet
- Development in Wastewater Treatment Research and Processes: Microbial Degradation of Xenobiotics Through Bacterial and Fungal Approach Maulin P. ShahDocument52 pagesDevelopment in Wastewater Treatment Research and Processes: Microbial Degradation of Xenobiotics Through Bacterial and Fungal Approach Maulin P. Shahchristy.delano512100% (6)
- Traditional Butter and Ghee Production, Processing and Handling in Ethiopia: A ReviewDocument11 pagesTraditional Butter and Ghee Production, Processing and Handling in Ethiopia: A ReviewAlexandra CîrsteaNo ratings yet
- Tabela Formulacoes FertilizantesDocument1 pageTabela Formulacoes FertilizantesGeraldo Aparecido SantosNo ratings yet
- Nanomedicine and Cancer: by Nicole Chia Poh HuiDocument9 pagesNanomedicine and Cancer: by Nicole Chia Poh HuiLas Cicas KchibiusaNo ratings yet
- Utilization of Palm Oil Mill Effluent - Avoiding CH Emission PT Agricinal, Bengkulu - IndonesiaDocument10 pagesUtilization of Palm Oil Mill Effluent - Avoiding CH Emission PT Agricinal, Bengkulu - Indonesiafebian_sam22No ratings yet
- Health Benefits and Possible Risks of Herbal Medicine: March 2016Document21 pagesHealth Benefits and Possible Risks of Herbal Medicine: March 2016Juan GomezNo ratings yet
- Pro Elite Analyzer Operation Manual 4001051 Rev A PDFDocument36 pagesPro Elite Analyzer Operation Manual 4001051 Rev A PDFintermountainwaterNo ratings yet
- S6900QLDocument4 pagesS6900QLmechkashanNo ratings yet
- Brochure - M Series en - Pompa OBLDocument6 pagesBrochure - M Series en - Pompa OBLAmrina RosyadaNo ratings yet
- "Multi Village Waste Management System Huligi": Waste Dumping Yard and Heaping of WasteDocument3 pages"Multi Village Waste Management System Huligi": Waste Dumping Yard and Heaping of WasteravibasarihalliNo ratings yet
- Japan Engine CatalgoDocument40 pagesJapan Engine CatalgoKarim Sowley DelgadoNo ratings yet
- Comparative Investigation On Tensile Performance of FRP Bars After Exposure To Water, Seawater, and Alkaline SolutionsDocument12 pagesComparative Investigation On Tensile Performance of FRP Bars After Exposure To Water, Seawater, and Alkaline Solutionssherif fodaNo ratings yet
- Fluconazole - Customer - Notification - Recall Communication - LetterDocument1 pageFluconazole - Customer - Notification - Recall Communication - Lettermohammed shaffi abdul rahmanNo ratings yet
- Balston Nitrogen GeneratorDocument10 pagesBalston Nitrogen GeneratorNguyễn NgọcNo ratings yet
- 3500-Mn Manganese (Editorial Revisions, 2011)Document3 pages3500-Mn Manganese (Editorial Revisions, 2011)Gustavo Baccho Jorge FilhoNo ratings yet
- Paints, CoatingsDocument9 pagesPaints, CoatingsazozinlcNo ratings yet
- Capillary Tube For Refrigeration and Air Conditioning SystemsDocument3 pagesCapillary Tube For Refrigeration and Air Conditioning SystemsIjazzzAliNo ratings yet