Professional Documents
Culture Documents
1 - Paper - Planta Piloto Absorcion H2S PDF
1 - Paper - Planta Piloto Absorcion H2S PDF
Uploaded by
Silas Calderon LuloOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1 - Paper - Planta Piloto Absorcion H2S PDF
1 - Paper - Planta Piloto Absorcion H2S PDF
Uploaded by
Silas Calderon LuloCopyright:
Available Formats
Absorption of H2S in NaOCl Caustic
Aqueous Solution
a a b
Luke Chen, James Huang, and Chen-Lu Yang
a
Department of Water Resources and Environmental Engineering, Tamkang University, Tamsui, Taipei Hsien, Taiwan
b
Hazardous Substance Management Research Center, New Jersey Institute of Technology, University Heights, Newark, NJ 07102
Pilot plant experimental data were collected to study the Aqueous amine solutions have been used commer-
feasibility of H S removal from air streams utilizing aqueous
2
cially since the 1930s to remove acid gases, such as
solutions. Solutions of NaOCl/NaOH were tested in a packed CO and H S, from a variety of hydrocarbon-based gas
2 2
streams, including natural gas, refinery gas, coke oven
bed scrubber and found to be effective. An efficiency of
gas, etc. Amine absorption units absorb the H S and 2
99.2% H S removal was achieved at a gas flow rate of 790
2
other acid compounds into an aqueous solution,
2
lb/ft -hr and liquid-gas ratio of 5.06. Sodium hydroxide was which is then heated. Heating regenerates the lean
found to be the active ingredient in the absorption process. A amine solution, while releasing a concentrated H S 2
minimum alkalinity of pH 11 in the scrubbing solution was gas stream. Generally, amine absorption units are lim-
required for the H S to be efficiently absorbed in the packed
2 ited to anaerobic gas streams since oxygen will oxi-
bed scrubber. For gas flow rates up to 2,100 lb/ft h r, the
2
dize the amines [4].
height of a transfer unit (HTU) varied from 1.8 ft to 2 ft with In the Claus Process, widely used in oil and natural
gas refining and processing facilities, one third of the
different proportions of NaOCl and NaOH in the solution.
H S is first oxidized to SO in a furnace. The SO and the
2 2 2
remaining H S then reacts in the furnace and in a series
2
INTRODUCTION of reactors to produce elemental sulfur. The overall
H y d rogen sulfide (H S) is produced in nature by
2
removal efficiency of a Claus Process is dependent on
anaerobic decomposition of sulfur-containing organic the number of catalytic reactors installed [5, 6, 7].
and inorganic matter. In recent years, industrial activi- The first commercial liquid oxidation process is the
ties have contributed substantially to H S emissions
2
Stretford Process, which uses an aqueous solution of
t h rough hydrogenation and hydro d e s u l f u r i z a t i o n sodium carbonate, sodium bicarbonate and
processes, and through anaerobic, thermal treatment anthraquinone disulfonic acid to dissolve oxygen in
processes, such as in coke ovens. No matter how it is the aqueous solution in order to oxidize the H S to 2
produced, H S poses a serious health risk, not to men-
2 sulfur. The reaction rate is slow. Consequently, alkali
tion an obnoxious odor. The human nose can detect vanadates are added to the solution to promote the
the “rotten egg” odor of H S at a concentration of 0.4
2 oxidation. Since vanadium is toxic, the main draw-
parts per billion (ppb). The maximum allowable back to the Stretford Process is that the process must
exposure for prolonged periods is 10 parts per million be designed to handle the sulfur cake, and the dis-
(ppm), the peak concentration for 10 minutes expo- charge solution [8]. The Stretford Process is modified
s u re is 50 ppm and exposure to concentrations to avoid the generation of toxic waste by using chelat-
greater than 300 ppm for 30 minutes is fatal [1]. ed iron to promote the reaction between H S and dis-
2
The two main purposes for removing H S from gas2 solved oxygen. Following the absorption of H S into 2
streams are to purify synthetic gas and to achieve air water and its subsequent ionization, sulfide anions are
pollutants control. For these goals, numerous method- oxidized to elemental sulfur. The accompanying reac-
ologies have been developed, and more than half a tion is the reduction of ferric ions to ferrous ions.
dozen have been demonstrated commercially. Among Adsorption for the removal of trace amounts of
these methods are amine absorption, alkaline salt pollutants has been in practice for years. For remov-
absorption, dry oxidation, liquid phase oxidation and ing H S from gas streams, activated carbon is the pre-
2
H S scavengers for gas purification and adsorption [2],
2
ferred adsorbent due to its high capacity [9, 10]. There
and caustic absorption and chemical oxidation for is evidence that, in the presence of oxygen, activated
end-of-pipe odor control [3]. carbon adsorbs H S and catalyzes the oxidation of the
2
Environmental Progress (Vol.20, No.3) October 2001 175
gas [11, 12]. Generally, activated carbon is used only will be released. The scenario may create an odor prob-
for removing H S from air streams, since the carbon
2 lem or, even worse, a hazardous condition. This is
would non-selectively adsorb most of the components because the reaction in Equation 1 is reversible. To pre-
present in an anaerobic stream. vent this from occurring, NaOCl is added to provide an
Caustic absorption systems are usually gro u p e d i r reversible reaction, as illustrated in Equation 2.
with chemical oxidation systems. However, the caustic Byproducts of the chemical oxidation are water soluble
absorption reaction is an equilibrium limited process and will accumulate in the scrubbing solution until it
while chemical oxidation processes in general are not. becomes saturated and salt precipitation occurs. As
In a caustic absorption system, the solution is main- shown in these equations, NaOH and NaOCl are contin-
tained at a high pH to enhance the absorption of H S. 2 uously consumed. This represents an operating cost that
To control the salt content of the scrubbing solution, a is directly proportional to the amount of H S being 2
portion is discharged from the system. If the pH of the removed.
spent scrubbing solution is allowed to decrease by
mixing it with other waste streams, the reaction is dri- CHEMICAL ABSORPTION IN A PACKED BED
ven back and H S is released to the atmosphere. This
2
Consider a packed bed with the following charac-
is the reason that the spent scrubbing solutions are teristics. The cross section is Ω and the diff e re n t i a l
classified as hazardous substances [13, 14]. volume in height d Z is Ωd Z. If the change in molar
Odor control in wastewater treatment plants is gener- flow rate F is neglected, the amount absorbed in sec-
ally accomplished by absorbing the malodorous com- tion dZ is -Fdy, which is equal to the absorption rate
pounds into aqueous solution and then chemically oxi- times the differential volume:
dizing them to innocuous, odorless compounds.
Although there are a variety of chemical oxidants avail- -Fdy = Kya (y-y*) ΩdZ (3)
able, such as chlorine (Cl ), ozone (O ), sodium
2 3
hypochlorite (NaOCl), potassium perm a n g a n a t e This equation is rearranged for integration by group-
( K M n O ), hydrogen peroxide (H O ) and ferric salt
3
4 2 2
ing the constant factors F, ΩdZ, and K a with dZ.
y
(Fe +), the most frequently used is a combination of
sodium hydroxide (NaOH) and sodium hypochlorite Ky aΩ ZT Ky aΩZ T a dy (4)
F ∫0 ∫b y − y ∗
[15, 16, 17, 18]because they are relatively inexpensive, dZ = =
readily available, and have a high oxidizing capacity. In F
this process, all of the reactions occur in the aqueous
phase in which the chemical oxidants are dissolved.
Consequently, the gaseous, odorous components must The equation for the column height can be written
contact the aqueous solution in a way that the odor as follows:
components will dissolve. Although counterc u r re n t
packed bed scrubbers are the most common configura- (5)
tion, spray towers, ejector venturi scrubbers, plate F
ZT = Ω
columns, mist scrubbers and even mobile-bed a dy
absorbers are used in this application [19, 20, 21, 22, 23,
Ky a ∫b y - y∗
24]. Odor control systems in wastewater tre a t m e n t
plants are designed to remove not only H S but also
2
other odorous components. The design is usually based
on an overall odor intensity.
A pilot plant program was initiated to test solutions The integral in Equation 5 represents the change in
of NaOH/NaOCl for their ability to absorb H S in a 2 gas phase concentration divided by the average driving
packed bed scrubber. The objective of this re s e a rc h force and is defined as the number of transfer units,
was to study H S as single target compound to obtain
2 NTU. The other part of Equation 5 has the unit of length
operating conditions and design parameters for pre- and is called the height of a transfer unit, HTU.
cise scrubber design. The chemical reaction in the liquid phase reduces
the equilibrium partial pressure of the solute over the
solution, which greatly increases the driving force for
THE CHEMISTRY
mass transfer. If the reaction is essentially irreversible
The actual absorption/oxidation reactions are quite
at absorption conditions, the equilibrium partial pres-
complex, however they can be represented by:
sure is zero, and the NTU can be calculated just from
the change in gas composition [25], where y* = 0.
H2S(g) + NaOH(aq) ↔ Na2S(aq) + 2H2O(l) (1)
dy y (6)
= 1n a
a
Na2S(aq) + 4NaOCl(aq) → Na2SO4(aq) + 4NaCl(aq) (2) NTU = ∫b
y yb
Equation 1 illustrates the reaction in a caustic absorp-
tion system. The reaction is driven to the right as the pH
A part of the re s e a rch described in this paper is
of the solution increases, i.e., as more caustic is added, directed at obtaining the HTU for H S absorption in an
2
and to the left as the pH is decreased. When the pH of NaOCl caustic aqueous solution.
the spent scrubbing solution is allowed to decrease, H S 2
176 October 2001 Environmental Progress (Vol.20, No.3)
removing entrained droplets from the gas stream. The
EXPERIMENTAL SECTION
The pilot plant scrubber consists of a gas blending entire column sits on top of a vessel which serves as
system, a gas scrubber, a chemical injection and con- the scrubbing solution reservoir.
trol system and an H S monitoring unit. Figure 1 is a
2
The concentrations of NaOH and NaOCl in the
schematic of the pilot plant. The gas blending system scrubbing solutions are monitored and controlled by a
is capable of producing a wide variety of gas compo- system of pH meter/metering pump, and oxidation
sitions by mixing air with high concentration H S from
2
reduction potential (ORP) meter/metering pump,
cylinders. The H S-containing air stream is then passed
2
respectively. A circulating pump withdraws scrubbing
through the scrubbing tower where H S is absorbed
2
solution from the reservoir and pumps it up to the top
and oxidized. Samples are taken from the inlet to be sprayed down on the packed bed, countercurrent
streams and effluents to determine the removal effi- to the gas flow. The rough pumping rate is controlled
ciency of H S and, through calculation, HTU.
2
by regulating the recirculating rate, with the final adjust-
ment being made at the Signet 5500 flow meter down-
Apparatus stream from the pump.
The gas blending system is capable of total flow
3
rates of 45 m /min (1600 cubic feet per minute, cfm). Analysis
The H S concentrations are regulated by injecting it
2
A Multi Rae PGM-50 H S analyzer is used to measure
2
from a 5% gas cylinder to the air stream. The whole the inlet and outlet concentrations of H S. The instru-
2
system is made of glass fiber re i n f o rced plastic ment utilizes the principle of UV photo ionization for
(FRP), including the blower, except for the H S injec-
2
detection and measurement of gas phase H S. During a
2
tion lines that are polypropylene tubing. After the normal operation, a continuous sample is drawn into
H S is injected into the air stream, the whole stream
2 the detector chamber by an internal pumping system.
is passed into a section of Te l l e rete Packings to The sample stream is metered and passed through the
achieve better mixing. The well mixed H S-contain-
2 particle filters before reaching the detector chamber,
ing air stream is then carried into the gas scrubber where the sample is exposed to UV light, which ionizes
where absorption and chemical reaction occur. the H S. An electric field drives ions to collect elec-
2
The packed bed scrubber is constructed of a 5 trodes, and generates a current corresponding to the
meter tall and 0.45 meter diameter polypropylene col- collection rate. An electrometer preamplifier is used to
umn with a 1.8 meter packed bed section randomly measure the current, and then sends the signal to an
packed with 3.25 inch, No. 2 K-type Tellerete Pack- e x t e rnal LCD display. The H S analyzer used in this
2
ings. The top of the column holds a demister head research has a detection range of 0 to 300 ppm with a
packed with No. 1 R-type Te l l e rete Packings for resolution of 0.1 ppm.
Figure 1. The schematic of the pilot plant gas scrubbing system.
Environmental Progress (Vol.20, No.3) October 2001 177
F i g u r e 2. The effect of inlet concentration on H 2 S Figure 3. The effect of NaOH on H2S absorption with
absorption in a packed bed scrubber. gas flow rates at 500 cfm and 700 cfm, and liquid
mass flow rate at 2,000 lb/ft2 hr.
Table 1. Experimental parameters.
Scrubber parameters
Column diameter (ID) m 0.45
Tower height m 5
Packing height m 1.8
Packing size (nominal) in 3.25
Gas parameters
Gas flow rate (standard) ft3/min, cfm 600
Gas mass flow rate lb/ft2 hr 1,500
Gas temperature (room) °C 25
Gas composition (H2S/air) ppm 200
Liquid parameters
Liquid mass flow rate lb/ft2 hr 2,000
Alkalinity (by NaOH) pH 11
ORP (by NaOCl) mV 450
Sodium Hydroxide
RESULTS AND DISCUSSION
A set of experiments were carried out with the pH
Parameters such as H S inlet concentrations, NaOH
2
held between 8 and 11.5 to determine its effect on H S 2
and NaOCl concentrations in the scrubbing solutions,
removal. The pH was controlled by pumping 45%
as well as gas and liquid flow rates were studied for
NaOH aqueous solution into the NaOCl-containing
their effect on H S removal. A range of operating con-
2
scrubbing solution. The results of these runs are given
ditions were established after these tests, and the
in Figure 3. The H S removal efficiency increased from
2
height of a transfer unit (HTU) was found to correlate
20% to 90% while the pH was increased from 8 to 11.5.
to gas flow rate. This correlation should be studied
The experiments were repeated at a higher gas flow
further before designing the full-scale scrubber.
rate. As expected, the H S removal efficiency at a flow
2
rate of 700 cfm was about 7% lower than that at 500
Hydrogen Sulfide cfm. Apparently, this is due to the shorter residence
A limited number of experiments were performed at time in the scrubber.
the conditions indicated in Table 1. The H S concentration
2
In view of these results, it was decided to increase
was varied from 50 to 200 ppm. The actual measurements the liquid rate to further reduce the outlet concentration
from these experiments are plotted in Figure 2. The data 2
of H S. The liquid rate was adjusted from 2000 lb/ft hr
2
indicate that H S is effectively absorbed in the NaOH
2 2
to 3000 lb/ft hr. Figure 4 shows the differences of H S 2
aqueous solution. However, the removal efficiency seems removal between these two runs. A 10% increase in
to be independent of the inlet concentrations of H S in the
2
removal efficiency was demonstrated across the whole
range of 50 to 200 ppm. range of pH from 8 to 12.
Sodium Hypochlorite
A set of runs were made under conditions identical
to those described in the previous section, except the
concentrations of NaOCl in the scrubbing solution
were adjusted to have ORPs of between 350 and 500
millivolts (mV). The ORPs were used to monitor and
178 October 2001 Environmental Progress (Vol.20, No.3)
Figure 4. The effect of NaOH on H2S absorption with F i g u re 5. The effect of NaOCl on H2S absorption in
liquid mass flow rates at 2,000 lb/ft 2 hr and 3,000 caustic aqueous scrubbing.
lb/ft2 hr.
control the concentration of NaOCl in the scrubbing of H S was sensitive to the L/G ratio only when pH
2
solution. The effect of ORP on H S removal are given
2 was low. There was a 10% to 20% diff e rence in H S 2
in Figure 5. The H S removal efficiency increases from
2 removal between the scrubbings at pH 10.5 and pH
87% to 93% with respect to an ORP increase from 350 11. It thus became apparent that only NaOH was the
mV to 500 mV. A slight enhancement of H S removal
2 active ingredient in this scrubbing process.
might have been due to the oxidation of sulfide ions
by the NaOCl in the scrubbing solution. Gas Flow Rate
When dissolved in water, NaOCl forms chlorine The liquid-gas mass ratio is the most important
(Cl ), hypochlorous acid (HOCl) and hypochlorite ions
2 parameter for the design of an absorption tower.
(OCl-) in the solution. White [26] provides equilibrium Thus, for a given gas flow, a reduction in liquid flow
data to determine the proportions of each component decreases the slope of the operating line. This, how-
as a function of pH. The presence of HOCl and OCl- e v e r, is not the case for chemical absorption. In a
were confirmed by continuous monitoring with a UV- chemical scrubber, H S is continuously removed by its
2
range photodiode detector in a previous study [27]. reaction with NaOH and NaOCl. There f o re, no H S 2
Figure 6 shows the equilibrium concentrations of Cl , 2 accumulates in the scrubbing solution, as stated in
HOCl and OCl- in the scrubbing solution, and the Equation 6. As long as a scrubbing solution is provid-
scrubber’s H S removal efficiency. At pH between 8.5
2 ed to cover a reasonable portion of the interfacial area
and 11.5, concentrations of the oxychlorine com- of the packings in the scrubber, liquid flow rate
pounds were unchanged, while H S removal increased
2 demonstrates a minimal effect on absorption efficien-
f rom 10% to 90% with the increase of alkalinity. cy. However, because H S removal is accomplished by
2
Apparently, none of the oxychlorine compounds are chemical reactions, residence time is an important
the active ingredient in this process. This is why the consideration. Thus, gas flow rate is expected to play
reaction in Equation 1 is proposed to be the rate a significant role in this process.
determining step of this process. A set of experiments was performed to determine
absorption rates of H S in NaOH/NaOCl aqueous
2
Liquid Gas Ratio scrubbing. The gas flow rate was varied from 300 cfm
A number of experiments were carried out at the to 900 cfm. Table 2 shows the result of reducing outlet
conditions described in Table 1 with NaOCl concentra- concentration of H S by increasing NaOCl to 0.2 M
2
2
tions of 0.003 and 0.006 molar (M). The gas flow rate
3
and liquid rate to 3,000 lb/ft hr. A 99.2% removal rate
was varied from 300 to 900 ft /min. The liquid rate was
2
was achieved. The absorption rate in terms of HTU
held constant at 3000 lb/ft hr. Thus, the practical range was calculated by plugging the packing height, and
of 1.25 to 3.75 liquid-to-gas mass ratio (L/G) was stud- inlet and outlet concentrations of H S into Equation 6.
2
ied. The actual measurements from these experiments The next set of experiments was designed to corre-
are plotted in Figure 7. The data indicate that the effec- late HTU to gas flow rate. The experiments were per-
tiveness of H S removal was almost constant with L/G. A
2 formed at the conditions indicated in Table 1 with the
minimum L/G of 1.5 was needed to have a stable H S 2 pH of 11 and 12. Figure 9 shows the HTU as a func-
removal. Figure 7 also shows a minimal difference of tion of gas flow rate. Theoretically, HTU is linear to
H S removal between the scrubbing solutions with dif-
2 gas flow rate. The slopes of the HTU plots decrease in
ferent NaOCl concentrations. the loading region because of the increase in interfa-
Two sets of experiments were run to determine the cial area. Figure 10 shows the same trend of the
effect of L/G on H S removal at pH 10.5 and 11. The
2 dependency of HTU on gas flow rate as NaOCl con-
results from these runs are plotted in Figure 8. The centrations varies. Apparently, the effect of pH on
results indicate that H S is absorbed more effectively
2 HTU is significant while the effect of NaOCl concen-
in solutions with high pH. The scrubbing effectiveness tration on HTU is minimal.
Environmental Progress (Vol.20, No.3) October 2001 179
Figure 6. Equilibrium concentrations of Cl2, HOCl and F i g u re 7. The effect of liquid-gas mass ratio on H 2S
OCl-, and absorption efficiency of H2S as a function absorption at NaOCl concentrations of 0.003 and
of pH in the scrubbing solution. 0.006 molar.
Table 2. Height of a transfer unit.
Gas mass rate L/G H2S inlet H2S outlet Removal HTU
(lb/ft2 hr) (ppm) (ppm) (%) (ft)
790 5.06 199 1.7 99.2 1.24
1053 3.8 198 2.3 98.8 1.33
1316 3.04 198 2.8 98.6 1.39
1579 2.53 197 3.2 98.4 1.43
1842 2.17 199 3.9 98.0 1.50
2105 1.9 196 4.7 97.6 1.58
2369 1.69 195 9.6 95.1 1.96
CONCLUSIONS LITERATURE CITED
The NaOH aqueous solution is effective for H S 2 1. National Institute for Occupational Safety and
removal in a packed bed scrubber. A 99.2% removal Health, NIOSH Pocket Guide to Chemical Hazards,
rate is achievable at a reasonable gas flow rate. The DHHS publication 85-114, 1987.
existence of NaOCl creates an irreversible reaction to 2. Kohl, A. and F. Riesenfeld, Gas Purification, 4th
prevent H S from accumulating in the solution and/or
2 Edition, Gulf Publishing, Houston, TX, 1985.
from being released to the atmosphere. 3. Rafson, H., Editor, Odor and VOC Control Hand-
The NaOH is the only chemical parameter which book, McGraw-Hill, New York, NY, 1996.
significantly affects the removal of H S from air
2 4. Kohl, A. and F. Riesenfeld, “Alkanolamines for
streams. The enhancement effect may be due to high Hydrogen Sulfide and Carbon Dioxide Removal,”
pH rather than specifically to NaOH. An alkalinity of Chapter 2, Gas Purification, 4th Edition, Gulf Pub-
pH 11 is essential to have an HTU in the range of one
2
lishing, Houston, TX, 1985.
to two feet for a gas flow rate between 700 lb/ft hr to
2
5. Kohl, A. and F. Riesenfeld, “Dry Oxidation
2,100 lb/ft hr. Processes for Hydrogen Sulfide Removal,” Chapter
As predicted by Equation 6, gas flow rate is the only 8, Gas Purification, 4th Edition, Gulf Publishing,
physical parameter which strongly influences the HTU. Houston, TX, 1985.
The slopes of the HTU plots decrease in the loading 6. Nedez, C. and J. Ray, “A New Claus Catalyst to
region because of the increase in interfacial area. Reduce Atmospheric Pollution,” Catalysis Today,
The ORP can be used to monitor and control the 27, 1996.
concentration of NaOCl in the scrubbing solution. 7. Suppiah, S. and D. Burn s, “Hydrogen Sulfide
Since the NaOCl does not react with H S directly, it
2 Oxidation Over Teflon Treated Activated Alumina
makes no sense to operate the scrubbing system at and Titanium Dioxide Catalysts,” Can. J. Chem.
high ORP. An ORP of 450 mV is adequate for a 99% Eng., 71, 1993.
removal, provided a pH of 12 is maintained. 8. Kohl, A. and F. Riesenfeld, “Liquid Phase Oxida-
tion Processes for Hydrogen Sulfide Removal,”
ACKNOWLEDGMENT Chapter 9, Gas Purification, 4th Edition, Gulf Pub-
The authors are grateful to Arthur Lee, President of lishing, Houston, TX, 1985.
Kunstoff Manufacturer Company, for support of this 9. Lever, J.P. and D.J. Jefferies, “Vapor Phase Filtra-
research. tion Using Activated Carbon,” Filtration and Sepa-
ration, 10, pp 707, 1993.
180 October 2001 Environmental Progress (Vol.20, No.3)
F i g u re 8. The effect of liquid-gas mass ratio on H 2S Figure 9. The effect of gas mass flow rate on HTU for
absorption at alkalinity of pH 10.5 and 11. H2S absorption in a packed bed scrubber at alkalinity
of pH 11 and 12.
10. Meeyoo, V., D. Trimm, and N. Cant, “Adsorp-
tion-Reaction Processes for the Removal of Hydro-
gen Sulfide from Gas Streams,” J. Chem. Te c h .
Biotechnol., 68, pp 411-416, 1997.
11. Ghosh, T.K. and E.L. To l l e f s o n, “Kinetics and
Mechanism of Hydrogen Sulfide Over Activated
Carbon in the Temperature Range of 125-200° C,”
Can. J. Chem. Eng., 64, pp 969, 1986.
12. Klein, J. and K. Henning, “Catalytic Oxidation
of Hydrogen Sulfide on Activated Carbons,” Fuel,
63, p 1064, 1984.
13. Water Environmental Federation and American
Society of Civil Engineers, “Odor Control in
Wastewater Treatment Plants,” WEF MOP 22,
ASCE Manuals and Reports on Engineering Prac-
tice, 82, 1995. Figure 10. The effect of gas mass flow rate on HTU for
14. American Water Works Association, “AWWA Stan- H2S absorption in a packed bed scrubber at NaOCl
d a rd for Caustic Soda,” ANSI/AW WA B501-88, concentrations of 0.003 and 0.006 molar.
Denver, CO, 1988.
15. Bonanni, E., “The Addition of Chemicals to Liq- 22. Stitt, E.H. and M.E. Fakley, “New Process for the
uid to Control Odors,” Section 8.1, Odor and VOC Abatement of Odorous and Low Level VOCs,”
C o n t rol Handbook, H. Rafson, Editor, McGraw- AIChE Spring National Meeting, Houston, TX,
Hill, New York, NY, 1996. March 20, 1996.
16. American Water Works Association, “AWWA Stan- 23. Stitt, E.H., K. Kelly, A.R. Elgood, and M.E. Fak-
dard for Ferrous Sulfate,” ANSI/AWWA B402-90, l e y, “Guarding Against Odors,” E n v i ro n m e n t a l
Denver, CO, 1990. Protection, January 1996.
17. American Water Works Association, “AWWA Stan- 24. Nagl, G., “Case History: Odor Control at a Water
d a rd for Hypochlorites,” ANSI/AW WA B300-87, Treatment Plant,” National Environmental Jour-
Denver, CO, 1987. nal, March/April 1996.
18. American Water Works Association, “AWWA Stan- 25. McCabe, W., J. Smith, and P. Harriott, U n i t
dard for Liquid Chlorine,” ANSI/AWWA B301-92, Operations of Chemical Engineering, 4th Edition,
Denver, CO, 1992. McGraw-Hill, New York, NY, 1985.
19. B o w k e r, R., “Chemical Scrubbing: Other 26. White, G.C., The Handbook of Water Chlorina-
Designs,” Section 8.6.3, Odor and VOC Contro l tion, 2nd Edition, Van Nostrand Reinhold Co.,
Handbook, H. Rafson, Editor, McGraw-Hill, New New York, NY, 1986.
York, NY, 1996. 27. Yang, C.-L., “Aqueous Absorption of Nitro g e n
20. Rafson, H., “Mist Scrubbing Technology, Recent Oxides Induced by Oxychlorine Compounds: A
Development and Current Practices in Odor Regula- P rocess Development Study for Flue Gas Tre a t-
tions, Control and Technology,” Transactions Air ment,” Doctoral thesis, New Jersey Institute of
and Waste Management Association, October 1989. Technology, 1994.
21. Waltrip, D. and E. Snyder, “Elimination of Odor
at Six Major Wastewater Treatment Plants,” J .
WPCA, 57, 10, pp 1027-1032, October 1995.
Environmental Progress (Vol.20, No.3) October 2001 181
You might also like
- Saturated and Unsaturated Solutions: Sci-BoxDocument9 pagesSaturated and Unsaturated Solutions: Sci-BoxNhet Ytienza50% (2)
- Adsorptive Desulfurization of DBT by Sewage Sludge - Derived Activated CarbonDocument9 pagesAdsorptive Desulfurization of DBT by Sewage Sludge - Derived Activated CarbonPhạm NgânNo ratings yet
- Safe Recovery of Platinum From Scrap AUTO CATALYTIC CONVERTERDocument6 pagesSafe Recovery of Platinum From Scrap AUTO CATALYTIC CONVERTERAFLAC ............86% (7)
- Sour Water Strippers ExposedDocument15 pagesSour Water Strippers Exposedreliability1100% (1)
- Experiment 2 - Preparation of Cis and Trans IsomerDocument7 pagesExperiment 2 - Preparation of Cis and Trans IsomerAbdulRahim059100% (3)
- 112 Experiment 3Document3 pages112 Experiment 3Amal ..No ratings yet
- Absorption of Hydrogen Sulfide and Methyl Mercaptan From Dilute Gas MixturesDocument7 pagesAbsorption of Hydrogen Sulfide and Methyl Mercaptan From Dilute Gas MixturesAndhikaAgraWisesaNo ratings yet
- Experimental Study of The Removal of Copper From Aqueous Solutions by Adsorption Using SawdustDocument8 pagesExperimental Study of The Removal of Copper From Aqueous Solutions by Adsorption Using SawdustchikubadgujarNo ratings yet
- Modelling of SO2 Absorption Into Aqueous NaHCO3 - Na2CO3Document12 pagesModelling of SO2 Absorption Into Aqueous NaHCO3 - Na2CO3Ariel TestinoNo ratings yet
- Designing Wet Scrubbers For SO2 AbsorptiDocument6 pagesDesigning Wet Scrubbers For SO2 AbsorptiDũng LêNo ratings yet
- Lixiviación Con Amoniaco en Reactor para El Secuestro de H2SDocument7 pagesLixiviación Con Amoniaco en Reactor para El Secuestro de H2SDeibyd ReyesNo ratings yet
- Theural Zeolite Ratio and AdsorbeDocument9 pagesTheural Zeolite Ratio and AdsorbeNick RonaldNo ratings yet
- 1 s2.0 0009250965801130 MainDocument5 pages1 s2.0 0009250965801130 MainreemNo ratings yet
- Pathways of Sulfide Oxidation by Haloalkaliphilic Bacteria in LimitedOxygen Gas Lift BioreactorsDocument6 pagesPathways of Sulfide Oxidation by Haloalkaliphilic Bacteria in LimitedOxygen Gas Lift BioreactorsJoel de la BarreraNo ratings yet
- Chemical Engineering Design and Analysis (049-076) PDFDocument28 pagesChemical Engineering Design and Analysis (049-076) PDFDavid Alyamir Triana GarciaNo ratings yet
- Study of A Mass Transfer-Reaction Model For SO Absorption Process Using LAS/H SO SolutionDocument6 pagesStudy of A Mass Transfer-Reaction Model For SO Absorption Process Using LAS/H SO SolutionFranck McNo ratings yet
- Treatment of Hydrogen Sulfide Gas Generated in Landfill SitesDocument7 pagesTreatment of Hydrogen Sulfide Gas Generated in Landfill SitesOmar MorónNo ratings yet
- Treatment of Hydrogen Sulfide Gas Generated in Landfill SitesDocument7 pagesTreatment of Hydrogen Sulfide Gas Generated in Landfill SitesOmar MorónNo ratings yet
- E21Document40 pagesE21ivarclNo ratings yet
- Lecture 16 Methane and Hydrates DM 2325Document59 pagesLecture 16 Methane and Hydrates DM 2325Top knotsNo ratings yet
- Investigación de Carbón Activo REV. 28 Sep - Es.enDocument4 pagesInvestigación de Carbón Activo REV. 28 Sep - Es.enGeomar VelezNo ratings yet
- Research ArticleDocument6 pagesResearch ArticleJoão Pedro GomesNo ratings yet
- Humic Acid From LigniteDocument14 pagesHumic Acid From Lignitekvsj2001No ratings yet
- W12 Control of SOxDocument69 pagesW12 Control of SOxNUR IZWANA BINTI IZAUDDINNo ratings yet
- Sulfide Oxydation With OxygenDocument10 pagesSulfide Oxydation With OxygenEdoardo ScaggianteNo ratings yet
- Removal of H2S With Metal Sulfates - NLDocument16 pagesRemoval of H2S With Metal Sulfates - NLEdoardo ScaggianteNo ratings yet
- Cell Chem Technol 1Document8 pagesCell Chem Technol 1Azhar AbbasNo ratings yet
- Hickman 1993Document4 pagesHickman 1993tieNo ratings yet
- Wu 2005Document9 pagesWu 2005Andres Mauricio Hernandez GarciaNo ratings yet
- Applied Energy: H. Selim, A. Al Shoaibi, A.K. GuptaDocument8 pagesApplied Energy: H. Selim, A. Al Shoaibi, A.K. GuptaAndrow Rafael Castro PerezNo ratings yet
- Adsorp and Absorp h2sDocument15 pagesAdsorp and Absorp h2sHVu NguyenNo ratings yet
- SUMSEM2016-17 CLE6010 ETH 1991 RM003 Art:10.1007/s40726-015-0015-ZDocument10 pagesSUMSEM2016-17 CLE6010 ETH 1991 RM003 Art:10.1007/s40726-015-0015-Zshaik mohammed ArshadNo ratings yet
- Methane Production: - Methanogenesis Substrates / Pathways Isotopic Studies Hydrogen CyclingDocument36 pagesMethane Production: - Methanogenesis Substrates / Pathways Isotopic Studies Hydrogen CyclingTemitope BelloNo ratings yet
- Pilot-Scale Biological Sulfide Oxidation Process For Treating Effluent From Rayon IndustryDocument14 pagesPilot-Scale Biological Sulfide Oxidation Process For Treating Effluent From Rayon IndustryMyo KyawNo ratings yet
- Chemical Engineering JournalDocument10 pagesChemical Engineering JournalGerson MartinezNo ratings yet
- Journal of Industrial and Engineering Chemistry: Gang Jin, Yujin Eom, Tai Gyu LeeDocument7 pagesJournal of Industrial and Engineering Chemistry: Gang Jin, Yujin Eom, Tai Gyu Leehaerul84No ratings yet
- Sigot 2016Document7 pagesSigot 2016QuiLeNo ratings yet
- Chemical Absorption of Carbon Dioxide With Triethanolamine in Non-Aqueous SolutionsDocument6 pagesChemical Absorption of Carbon Dioxide With Triethanolamine in Non-Aqueous SolutionsMuhammad Arsalan AshrafNo ratings yet
- Palynomorph Extraction From Peat, Lignite and CoalDocument5 pagesPalynomorph Extraction From Peat, Lignite and CoalGhaier KazmiNo ratings yet
- Kinetics and Mechanism Study On Chlorine Dioxide Generation With Hydrogen PeroxideDocument5 pagesKinetics and Mechanism Study On Chlorine Dioxide Generation With Hydrogen PeroxideirNo ratings yet
- Jsir 66 (2) (2007) 170-177Document8 pagesJsir 66 (2) (2007) 170-177Aditya sharmaNo ratings yet
- Mansfield SelectionFullScaleUse 1992Document9 pagesMansfield SelectionFullScaleUse 1992ingnatatlNo ratings yet
- PeroxideTriangleDiagrams TAPPIDocument8 pagesPeroxideTriangleDiagrams TAPPIEugênia PheganNo ratings yet
- Hydrogen Production From Water by Using Hydrogen Sulfide As Reducing Agent in Hydrothermal ReactionsDocument4 pagesHydrogen Production From Water by Using Hydrogen Sulfide As Reducing Agent in Hydrothermal ReactionsLukman HakimNo ratings yet
- 1998 - Wet Oxidation of Acetic Acid by Hydrogen Oxidation Catalyzed by Transition Metal-Exchange NaY Zeolites - LarachiDocument4 pages1998 - Wet Oxidation of Acetic Acid by Hydrogen Oxidation Catalyzed by Transition Metal-Exchange NaY Zeolites - Larachipetru apopeiNo ratings yet
- Study of Mercury Absorption and Desorption On Sulfur Impregnated CarbonDocument6 pagesStudy of Mercury Absorption and Desorption On Sulfur Impregnated Carbonamir.m.norouzi95No ratings yet
- Huang2015 Article ANovelProcessToRecoverSulfurIn PDFDocument7 pagesHuang2015 Article ANovelProcessToRecoverSulfurIn PDFHaNo ratings yet
- Removal of Phosphate From Aqueous Solutions Using Calcined Metal Hydroxides Sludge Waste Generated From ElectrocoagulationDocument8 pagesRemoval of Phosphate From Aqueous Solutions Using Calcined Metal Hydroxides Sludge Waste Generated From ElectrocoagulationLamine tech & scienceNo ratings yet
- JPPS 0209 Zhen GalleyDocument8 pagesJPPS 0209 Zhen GalleyGregorio ValeroNo ratings yet
- Direct Synthesis of Formic Acid From Carbon Dioxide by Hydrogenation in Acidic MediaDocument8 pagesDirect Synthesis of Formic Acid From Carbon Dioxide by Hydrogenation in Acidic MediaWilly ChandraNo ratings yet
- Mathematical Modeling of Hydrogen Production Process by Pressure Swing Adsorption MethodDocument12 pagesMathematical Modeling of Hydrogen Production Process by Pressure Swing Adsorption MethodEvminidaNo ratings yet
- IBA Carbonation 2006 B128 73 RefDocument7 pagesIBA Carbonation 2006 B128 73 RefMike LiuNo ratings yet
- Anaerobic Treatment of Sulphate-Rich WastewatersDocument12 pagesAnaerobic Treatment of Sulphate-Rich WastewatersEduardo Serrano perezNo ratings yet
- Precipitation of Metals in A Fixed-Bed Sulphate-Reducing Reactor Under Theoretical Stoichiometric Lactate/sulphate RatioDocument6 pagesPrecipitation of Metals in A Fixed-Bed Sulphate-Reducing Reactor Under Theoretical Stoichiometric Lactate/sulphate RatioIoannis KapageridisNo ratings yet
- EPA02286 European Chemical Bulletin 2014-01-108-112Document5 pagesEPA02286 European Chemical Bulletin 2014-01-108-112Anonymous cgKtuWzNo ratings yet
- Review of H2S Sorbents at Low-Temperature Desulfurization of BiogasDocument7 pagesReview of H2S Sorbents at Low-Temperature Desulfurization of BiogasHamed HpNo ratings yet
- A Novel Process To Recover Sulfur in Aqueous Phase Under Ambient Condition - SpringerLinkDocument16 pagesA Novel Process To Recover Sulfur in Aqueous Phase Under Ambient Condition - SpringerLinkmodikiritNo ratings yet
- Odour Removal in Leather TanneryDocument5 pagesOdour Removal in Leather TanneryterumistarNo ratings yet
- 6283 48058 1 PBDocument14 pages6283 48058 1 PBWajeeh khanNo ratings yet
- Sulfur Recovery Units (SRU)Document5 pagesSulfur Recovery Units (SRU)Splish SplashNo ratings yet
- Preparation and Characterization of Charcoals That Contain Dispersed Aluminum Oxide As Adsorbents For Removal of Fluoride From Drinking WaterDocument11 pagesPreparation and Characterization of Charcoals That Contain Dispersed Aluminum Oxide As Adsorbents For Removal of Fluoride From Drinking WaterCamila NevesNo ratings yet
- A Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidFrom EverandA Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidNo ratings yet
- 聚甲氧基二甲醚的合成及其物理化学性质表征 英文 康美荣Document9 pages聚甲氧基二甲醚的合成及其物理化学性质表征 英文 康美荣董芳儒No ratings yet
- Chapter3Document98 pagesChapter3Dani elbannaNo ratings yet
- Chemistry Acids Bases and SaltsDocument6 pagesChemistry Acids Bases and Saltssiba padhyNo ratings yet
- Haloalkanes WorksheetDocument6 pagesHaloalkanes WorksheetraginieraNo ratings yet
- Biomaterial - HydrogelDocument75 pagesBiomaterial - HydrogelMauhibahYumnaNo ratings yet
- TCCS ExamplesDocument757 pagesTCCS ExamplesBruno FreitasNo ratings yet
- Quantum Mechanics II - Homework 2Document6 pagesQuantum Mechanics II - Homework 2Ale GomezNo ratings yet
- LCAO MO Theory Illustrated by Its Application To H2Document8 pagesLCAO MO Theory Illustrated by Its Application To H2maugonzalezsuarezNo ratings yet
- Adge CompiledDocument18 pagesAdge CompiledNovelyn LumboyNo ratings yet
- Статья - 1 фнт НабойченкоDocument8 pagesСтатья - 1 фнт НабойченкоОльга НабойченкоNo ratings yet
- Effective Removal of Toxic Heavy Metal Ions From Aqueous Solution by CaCO3 MicroparticlesDocument14 pagesEffective Removal of Toxic Heavy Metal Ions From Aqueous Solution by CaCO3 MicroparticlesDiego VeneuNo ratings yet
- Selection GuideDocument6 pagesSelection GuideJoey DunnNo ratings yet
- Chapter 1 Introduction To Engineering Principles and Units PDFDocument26 pagesChapter 1 Introduction To Engineering Principles and Units PDFAzam MuddinNo ratings yet
- Synthesis and Optical Properties of Uantum-Size Metal Sulfide Particles in Aqueous SolutionDocument4 pagesSynthesis and Optical Properties of Uantum-Size Metal Sulfide Particles in Aqueous SolutionANGIE PAOLA RODELO PANZANo ratings yet
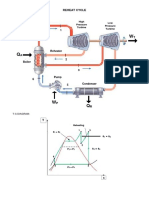
- Reheat Cycle: Schematic DiagramDocument2 pagesReheat Cycle: Schematic DiagramDave BalladoNo ratings yet
- Recent Trends in Non-Traditional Machining Processes: Unit - 5Document12 pagesRecent Trends in Non-Traditional Machining Processes: Unit - 5DISHA VNo ratings yet
- QA8 QC For CapsuleDocument33 pagesQA8 QC For Capsulealshhatmhmdahmd153No ratings yet
- PRISMsvvDocument20 pagesPRISMsvvJatin sutharNo ratings yet
- Exam Style Answers 19 Asal Physics CBDocument2 pagesExam Style Answers 19 Asal Physics CBAnshul ShahNo ratings yet
- 9 - PDFsam - Electricity and Electronics For HVACDocument1 page9 - PDFsam - Electricity and Electronics For HVACwarlen11No ratings yet
- Fluent 13.0 Lecture09-Physics PDFDocument74 pagesFluent 13.0 Lecture09-Physics PDFChristian RodriguezNo ratings yet
- Halogenoalkanes NotesDocument5 pagesHalogenoalkanes NotesAgustina Tedja100% (1)
- 2011f F II.3Document19 pages2011f F II.3arleneNo ratings yet
- Entropy: Shahroze UmarDocument24 pagesEntropy: Shahroze UmarHusnain AliNo ratings yet
- Chemical Conversion of Steel Mill Gases To Urea - An Analysis of Plant CapacityDocument8 pagesChemical Conversion of Steel Mill Gases To Urea - An Analysis of Plant CapacityNestor TamayoNo ratings yet
- Example 11 RefrigerationDocument3 pagesExample 11 RefrigerationSantosh RathodNo ratings yet
- R3910 Scheme of Valuation/Answer Key: Apj Abdul Kalam Technological UniversityDocument7 pagesR3910 Scheme of Valuation/Answer Key: Apj Abdul Kalam Technological UniversityALAN JOYNo ratings yet