Professional Documents
Culture Documents
Vaporization Surface Liquid: Evaporation Is A Type of
Vaporization Surface Liquid: Evaporation Is A Type of
Uploaded by
Victoria CebanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Vaporization Surface Liquid: Evaporation Is A Type of
Vaporization Surface Liquid: Evaporation Is A Type of
Uploaded by
Victoria CebanCopyright:
Available Formats
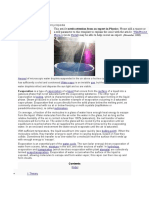
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the
gas phase.[1] The surrounding gas must not be saturated with the evaporating substance. When
the molecules of the liquid collide, they transfer energy to each other based on how they collide
with each other. When a molecule near the surface absorbs enough energy to overcome
the vapor pressure, it will escape and enter the surrounding air as a gas.[2] When evaporation
occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid,
resulting in evaporative cooling.[3]
On average, only a fraction of the molecules in a liquid have enough heat energy to escape from
the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of
the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until
the surrounding air is saturated.
Evaporation is an essential part of the water cycle. The sun (solar energy) drives evaporation of
water from oceans, lakes, moisture in the soil, and other sources of water. In hydrology,
evaporation and transpiration (which involves evaporation within plant stomata) are collectively
termed evapotranspiration. Evaporation of water occurs when the surface of the liquid is
exposed, allowing molecules to escape and form water vapor; this vapor can then rise up and
form clouds. With sufficient energy, the liquid will turn into vapor.
You might also like
- EvaporationDocument2 pagesEvaporationvijay kumar honnaliNo ratings yet
- Describe Evaporation in Terms of The Escape of More Energetic Molecules From The Surface of A LiquidDocument2 pagesDescribe Evaporation in Terms of The Escape of More Energetic Molecules From The Surface of A LiquidRoyston EbenezerNo ratings yet
- The Earth and Its AtmosphereDocument28 pagesThe Earth and Its AtmosphereselahNo ratings yet
- Best AnswerDocument2 pagesBest Answermanasa ranaNo ratings yet
- Evaporation - WikDocument18 pagesEvaporation - WikPrince SinghNo ratings yet
- Evaporation - WikipediaDocument23 pagesEvaporation - WikipediaBashiir NuurNo ratings yet
- Earth Science ReportDocument2 pagesEarth Science ReportJayve OlimbaNo ratings yet
- EvaporationDocument8 pagesEvaporationNikhil SajiNo ratings yet
- Unit 2: Micro EnvironmentDocument8 pagesUnit 2: Micro EnvironmentHasini KadaruNo ratings yet
- Evaporation: From Wikipedia, The Free EncyclopediaDocument6 pagesEvaporation: From Wikipedia, The Free EncyclopediaSubbaReddyNo ratings yet
- Hydrology (Student Module) - Chapter 1.1Document27 pagesHydrology (Student Module) - Chapter 1.1Jake CanlasNo ratings yet
- Chapter 3Document12 pagesChapter 3Jaypee MelendezNo ratings yet
- Factors Affecting The Rate of EvaporizationDocument4 pagesFactors Affecting The Rate of EvaporizationJoyme Ann SantosNo ratings yet
- Jsaer2016 03 01 51 54Document4 pagesJsaer2016 03 01 51 54jsaereditorNo ratings yet
- Ged102 MeteorologyDocument7 pagesGed102 MeteorologyTadz-alniza JajiNo ratings yet
- Earth SystemDocument6 pagesEarth SystemDwayne Ashley SilveniaNo ratings yet
- Evs - Final Unit 1 - IntroductionDocument96 pagesEvs - Final Unit 1 - IntroductionJay Guruprasad KanitkarNo ratings yet
- CEG503SurfaceWaterHydrology-Lecture 4 Evaporation 2018semesterDocument16 pagesCEG503SurfaceWaterHydrology-Lecture 4 Evaporation 2018semesterOMOGBEHIN SEUNNo ratings yet
- Theory: Evaporation, A Type ofDocument3 pagesTheory: Evaporation, A Type ofHarisma NugrahaNo ratings yet
- Convection: Convection Is The Concerted, CollectiveDocument12 pagesConvection: Convection Is The Concerted, CollectivebekkuNo ratings yet
- Evaporation ControlDocument5 pagesEvaporation Controlvikrambhadarka1738No ratings yet
- Atmosphere: Phase I MeteorologyDocument15 pagesAtmosphere: Phase I MeteorologyNelum PereraNo ratings yet
- The AtmosphereDocument4 pagesThe AtmosphereNina CervantesNo ratings yet
- GME Imp QuestionDocument8 pagesGME Imp QuestionSkaria ParackalNo ratings yet
- Chapter 3 EvapotranspirationDocument20 pagesChapter 3 EvapotranspirationnimcanNo ratings yet
- Earths Subsystems - Learning MaterialDocument3 pagesEarths Subsystems - Learning MaterialJA Berzabal100% (1)
- Explain The Earth Climatic Systems, Its Components and Their Interaction in DetailDocument16 pagesExplain The Earth Climatic Systems, Its Components and Their Interaction in DetailAbdur RaufNo ratings yet
- 19 - 50 - 0412 SeminarDocument30 pages19 - 50 - 0412 SeminarSeye KareemNo ratings yet
- Earth Processes Atmosphere NotesDocument6 pagesEarth Processes Atmosphere NotesMuhammad SabeehNo ratings yet
- 04a. Hydrological and Atmospheric ProcessesDocument2 pages04a. Hydrological and Atmospheric ProcessesEileen TranNo ratings yet
- (Template) Geography 2Document79 pages(Template) Geography 2Mia Mones Lunsayan BrionesNo ratings yet
- Earth and Earth SystemsDocument4 pagesEarth and Earth SystemsMedicareMinstun ProjectNo ratings yet
- Civ300 NotesDocument5 pagesCiv300 Notessupermusician12345No ratings yet
- Environmental StudiesDocument30 pagesEnvironmental StudiesMuskan NarulaNo ratings yet
- HydrologyDocument3 pagesHydrologyJohn Dexter Lanot100% (1)
- Module 2 Earth Science 2021 2022Document3 pagesModule 2 Earth Science 2021 2022Razen SisonNo ratings yet
- Group 1 ReportDocument19 pagesGroup 1 ReportKimmy Jane FloresNo ratings yet
- Chapter 4 - Telangana 9th GeographyDocument4 pagesChapter 4 - Telangana 9th GeographyDhatri Subasri Navya KNo ratings yet
- Week 2 Module 2 Els Lecture Earth SubsytemDocument9 pagesWeek 2 Module 2 Els Lecture Earth SubsytemScore ExE100% (1)
- Insolation and Heat Balance of The EarthDocument10 pagesInsolation and Heat Balance of The EarthDevraj H SNo ratings yet
- Chapter 1 NotesDocument4 pagesChapter 1 NotesLena RobertsNo ratings yet
- Meteorology:: Atmospher and Ocean ClimateDocument28 pagesMeteorology:: Atmospher and Ocean ClimateDanny Satria100% (1)
- Weather Basics NotesDocument24 pagesWeather Basics NotesAna Bella RosarioNo ratings yet
- Earth SystemsDocument13 pagesEarth SystemsJoshua Sambrano BelandresNo ratings yet
- RainPhy JSAER0301007Document5 pagesRainPhy JSAER0301007Nasar UllahNo ratings yet
- Unit Two Components of The Environment and Their InteractionsDocument62 pagesUnit Two Components of The Environment and Their InteractionsDn Yossef MebrieNo ratings yet
- Diurnal Energy Budget Refers To The Balance Between The Amount of Energy Received by The EarthDocument5 pagesDiurnal Energy Budget Refers To The Balance Between The Amount of Energy Received by The EarthTeernce RugoyiNo ratings yet
- Geochemical Cycles NotesDocument6 pagesGeochemical Cycles NotesPerlievic TesoroNo ratings yet
- UNIT 1 Introduction To Earth's AtmosphereDocument9 pagesUNIT 1 Introduction To Earth's AtmosphereRegved PandeNo ratings yet
- LA ConvectionDocument4 pagesLA ConvectionmaanchielynarancesNo ratings yet
- EVS, Unit-IDocument49 pagesEVS, Unit-ISayeda AiennNo ratings yet
- Environmental Science FinalDocument43 pagesEnvironmental Science FinalMuhammadMansoorGoharNo ratings yet
- Topic HydrologyDocument6 pagesTopic HydrologyvictorNo ratings yet
- 2 Earth's Climate System Sun and Components FBDocument8 pages2 Earth's Climate System Sun and Components FBbggcw6w5qxNo ratings yet
- 16 DefinitionsDocument1 page16 Definitionssibi RajNo ratings yet
- Earth SubsystemDocument53 pagesEarth SubsystemMarcellie Cruz Canja0% (1)
- Environmental Science: 1. What Is The Sequence of Strata of Atmosphere and On What Factors Does It Depends?Document15 pagesEnvironmental Science: 1. What Is The Sequence of Strata of Atmosphere and On What Factors Does It Depends?Ali HussainNo ratings yet
- Geol-227 Ch-1 - A Changing PlanetDocument15 pagesGeol-227 Ch-1 - A Changing Planetcelinaradwan16No ratings yet
- Unit 1 Introduction To Environmental StudiesDocument7 pagesUnit 1 Introduction To Environmental Studiesanirudh shirkeNo ratings yet
- Sir William Thomson, 1st Baron Kelvin,: OM Gcvo PC PRS FrseDocument1 pageSir William Thomson, 1st Baron Kelvin,: OM Gcvo PC PRS FrseVictoria CebanNo ratings yet
- Portuguesa: Portugal (Document1 pagePortuguesa: Portugal (Victoria CebanNo ratings yet
- Peñón de Vélez de La GomeraDocument1 pagePeñón de Vélez de La GomeraVictoria CebanNo ratings yet
- The English Constitution: by Whom? Citation NeededDocument1 pageThe English Constitution: by Whom? Citation NeededVictoria CebanNo ratings yet
- ScandinavianDocument1 pageScandinavianVictoria CebanNo ratings yet
- Russian (русский язык,Document1 pageRussian (русский язык,Victoria CebanNo ratings yet
- Order of Merit Edward VII Elizabeth II Commonwealth Realms Post-Nominal LettersDocument1 pageOrder of Merit Edward VII Elizabeth II Commonwealth Realms Post-Nominal LettersVictoria CebanNo ratings yet
- Soviet Union,: Glasnost PerestroikaDocument1 pageSoviet Union,: Glasnost PerestroikaVictoria CebanNo ratings yet
- Elizabeth II (Elizabeth Alexandra Mary Born 21 April 1926) : Annus HorribilisDocument1 pageElizabeth II (Elizabeth Alexandra Mary Born 21 April 1926) : Annus HorribilisVictoria CebanNo ratings yet
- Surface StressDocument1 pageSurface StressVictoria CebanNo ratings yet
- Sovereign State Queen Elizabeth II Constitutional Monarch Head of State KingdomDocument1 pageSovereign State Queen Elizabeth II Constitutional Monarch Head of State KingdomVictoria CebanNo ratings yet
- Incompressible Fluid The Four Fundamental States of Matter Solid Gas Plasma Intermolecular Bonds Able To FlowDocument1 pageIncompressible Fluid The Four Fundamental States of Matter Solid Gas Plasma Intermolecular Bonds Able To FlowVictoria CebanNo ratings yet
- De España) ,: Spain (Document1 pageDe España) ,: Spain (Victoria CebanNo ratings yet
- Josiah Willard GibbsDocument1 pageJosiah Willard GibbsVictoria CebanNo ratings yet
- Surface Tension Is The Tendency Of: Liquid Surface Area Water StridersDocument1 pageSurface Tension Is The Tendency Of: Liquid Surface Area Water StridersVictoria CebanNo ratings yet
- AtlanticDocument1 pageAtlanticVictoria CebanNo ratings yet
- Greek Water Planet Minor Planet Natural Satellite Seafloor Spreading Continental DriftDocument1 pageGreek Water Planet Minor Planet Natural Satellite Seafloor Spreading Continental DriftVictoria CebanNo ratings yet
- Ancient Greek Includes The Forms of TheDocument1 pageAncient Greek Includes The Forms of TheVictoria CebanNo ratings yet
- AsiaDocument1 pageAsiaVictoria CebanNo ratings yet
- Greek (Greek: Ελληνικά,: Elliniká) is an independent branch of theDocument1 pageGreek (Greek: Ελληνικά,: Elliniká) is an independent branch of theVictoria CebanNo ratings yet
- World OceanDocument1 pageWorld OceanVictoria CebanNo ratings yet
- The Adventures of Sherlock Holmes: Arthur Conan DoyleDocument162 pagesThe Adventures of Sherlock Holmes: Arthur Conan DoyleVictoria CebanNo ratings yet