Professional Documents
Culture Documents
Realation Between K and K With Their Units
Realation Between K and K With Their Units
Uploaded by
VIKRAM KUMAR0 ratings0% found this document useful (0 votes)
8 views2 pagesThe document discusses the relationship between equilibrium constants KP and KC. KP is the equilibrium constant expressed in terms of partial pressures for gases, while KC is expressed in terms of molar concentrations for solutions. The document shows that KP and KC are related based on the ideal gas law, and provides the units for each constant and their possible positive or negative values depending on the reaction.
Original Description:
Dissociation of Ammonium Hydrosulphide
Original Title
Where Value
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the relationship between equilibrium constants KP and KC. KP is the equilibrium constant expressed in terms of partial pressures for gases, while KC is expressed in terms of molar concentrations for solutions. The document shows that KP and KC are related based on the ideal gas law, and provides the units for each constant and their possible positive or negative values depending on the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
8 views2 pagesRealation Between K and K With Their Units
Realation Between K and K With Their Units
Uploaded by
VIKRAM KUMARThe document discusses the relationship between equilibrium constants KP and KC. KP is the equilibrium constant expressed in terms of partial pressures for gases, while KC is expressed in terms of molar concentrations for solutions. The document shows that KP and KC are related based on the ideal gas law, and provides the units for each constant and their possible positive or negative values depending on the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
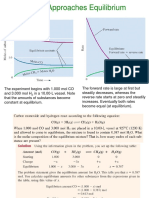
Where ‘K’ is equilibrium constant
As stated above the active mass is expressed in terms of mole/ litre (for solutions) or
partial pressure (for gases). Similarly, equilibrium constant K is expressed as for
solutions or gases, respectively.
For the above reaction, KP and KC are related as follows:
Since, PV = nRT
Let,
Where R = gas constant, T = absolute temperature and = [No. of moles of products
- No. of moles of reactants].
Realation between kp and kc with their units
Value of
Relation
Units of : No units
Units of : No units
Value of : > 0 or (+) Ve
Relation
Units of :
Units of :
Value of : < 0 or (-) ve
Relation
Units of :
Units of :
Related posts:
1. Law of Mass Action wor
You might also like
- Assignment - 4 1. Recitation Problems 1.1.1. Three Computers, A, B, and C, Have The Numerical Features Listed BelowDocument15 pagesAssignment - 4 1. Recitation Problems 1.1.1. Three Computers, A, B, and C, Have The Numerical Features Listed BelowVIKRAM KUMAR100% (1)
- Xin Yao ITSS 3300 07/04/2020Document10 pagesXin Yao ITSS 3300 07/04/2020VIKRAM KUMARNo ratings yet
- Regression StatisticsDocument1 pageRegression StatisticsVIKRAM KUMARNo ratings yet
- TAL Distributors Chapter 5Document7 pagesTAL Distributors Chapter 5VIKRAM KUMARNo ratings yet
- Using Complete Sentences Written in Your Own Words, Explain The Meaning of The FollowingDocument6 pagesUsing Complete Sentences Written in Your Own Words, Explain The Meaning of The FollowingVIKRAM KUMARNo ratings yet
- Supply Chain Management: From Vision To Implementation: by Stanley Fawcett, Lisa Ellram, and Jeffrey OgdenDocument8 pagesSupply Chain Management: From Vision To Implementation: by Stanley Fawcett, Lisa Ellram, and Jeffrey OgdenVIKRAM KUMARNo ratings yet
- 1Document2 pages1VIKRAM KUMARNo ratings yet
- It 2035C Network Infrastructure Management: C1 Design Scenario: ElectromycycleDocument2 pagesIt 2035C Network Infrastructure Management: C1 Design Scenario: ElectromycycleVIKRAM KUMARNo ratings yet
- HW 2. Chapter 4 (3, 4, 8, 9, 10)Document10 pagesHW 2. Chapter 4 (3, 4, 8, 9, 10)VIKRAM KUMAR0% (1)
- Hilts 105Document277 pagesHilts 105Wesley CheungNo ratings yet
- Chemical EqulibriumDocument23 pagesChemical Equlibriumiqbal-cheNo ratings yet
- Chapter Thirteen Chemical Equilibrium: For ReviewDocument42 pagesChapter Thirteen Chemical Equilibrium: For ReviewDedi WahyudinNo ratings yet
- Chemical Equilibrium - Types, Principles and Laws of EquilibriaDocument13 pagesChemical Equilibrium - Types, Principles and Laws of EquilibriaRafael TayoNo ratings yet
- CHM 111 Notes - 2021 - 2022Document19 pagesCHM 111 Notes - 2021 - 2022j9927091No ratings yet
- Chemical Equilibrium-UAPDocument26 pagesChemical Equilibrium-UAPMd. Mujahid HasanNo ratings yet
- Short Notes On EquilibriumDocument2 pagesShort Notes On EquilibriumRichard VincentNo ratings yet
- Chemical KineticsDocument10 pagesChemical KineticsTanayaa PatilNo ratings yet
- Chemical Equlibrium (Autosaved)Document21 pagesChemical Equlibrium (Autosaved)iqbal-cheNo ratings yet
- Chap 13Document41 pagesChap 13Shamaas HussainNo ratings yet
- Equilibrium ReactionDocument2 pagesEquilibrium ReactionAlif Nuzulul HidayatNo ratings yet
- Genchem Reviewer (2nd Grading)Document27 pagesGenchem Reviewer (2nd Grading)assassin1252005No ratings yet
- Important Equations For EngineersDocument4 pagesImportant Equations For Engineersmuhammad IrfanNo ratings yet
- Chemical Reaction Engineering Prof. Jayantmodak Department of Chemical Engineering Indian Institute of Science, BangaloreDocument19 pagesChemical Reaction Engineering Prof. Jayantmodak Department of Chemical Engineering Indian Institute of Science, BangaloreDeepak PandeyNo ratings yet
- Chemical Equilibrium NotesDocument11 pagesChemical Equilibrium NotesManu NathNo ratings yet
- Gas Behaviour EOSDocument59 pagesGas Behaviour EOSMurugavel ChandranNo ratings yet
- Chemical Kinetics 24-25Document12 pagesChemical Kinetics 24-25moiiifitbituserNo ratings yet
- Phase EquilibriaDocument21 pagesPhase EquilibriasuperchellyNo ratings yet
- Unit 2 Gas Laws and Power CyclesDocument103 pagesUnit 2 Gas Laws and Power CyclesNishad BhavsarNo ratings yet
- Chapter 07Document9 pagesChapter 07zahidNo ratings yet
- Physical Chemistry 2Document30 pagesPhysical Chemistry 2Michelle MenciasNo ratings yet
- GPSA Engineering Data Book 14th Edition: Revision Date Reason (S) For RevisionDocument31 pagesGPSA Engineering Data Book 14th Edition: Revision Date Reason (S) For RevisionAsad KhanNo ratings yet
- SCHX1014 - Chemical Engineering Thermodynamics - Unit 3Document17 pagesSCHX1014 - Chemical Engineering Thermodynamics - Unit 3Shanmuga PriyaNo ratings yet
- 6 ArrheniusDocument4 pages6 ArrheniushixzaNo ratings yet
- Chemical Equilibrium - Law of Mass Action and Equilibrium ConstantDocument28 pagesChemical Equilibrium - Law of Mass Action and Equilibrium ConstantJoshua LaBordeNo ratings yet
- Chemical EquilibriummDocument12 pagesChemical EquilibriummRaj bhaskarNo ratings yet
- Chemical Equilibrium: A+B C +DDocument14 pagesChemical Equilibrium: A+B C +DMD JAHEDUL ISLAMNo ratings yet
- Chapter 15Document32 pagesChapter 15Dana CapbunNo ratings yet
- Chapter 5 - Nahid - July 2017Document32 pagesChapter 5 - Nahid - July 2017Abdul BariNo ratings yet
- Section 25Document31 pagesSection 25rkm_rkmNo ratings yet
- Chemical Equilibrium .PresentationDocument17 pagesChemical Equilibrium .PresentationtalhawasimNo ratings yet
- Chapter 2 ThermoDocument5 pagesChapter 2 ThermoAlimah AzeliNo ratings yet
- Edexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaDocument6 pagesEdexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaMer CyNo ratings yet
- PCE Lecture 5 1 CRE IntroductionDocument20 pagesPCE Lecture 5 1 CRE IntroductionCH21B027 MEGAVARSHINI MNo ratings yet
- Vidya EquilibriumDocument65 pagesVidya EquilibriumNarendraNo ratings yet
- Section 25Document31 pagesSection 25Nadia Rahmeita PrasantiNo ratings yet
- Physical Chemistry ThermodinamicsDocument19 pagesPhysical Chemistry ThermodinamicsAdilla Rizka YonitaNo ratings yet
- CH 7 - Chemical KineticsDocument60 pagesCH 7 - Chemical KineticsCharbel RahmeNo ratings yet
- Environmental Chemistry and Microbiology: NptelDocument57 pagesEnvironmental Chemistry and Microbiology: NptelAbhijit NathNo ratings yet
- Articulo Equilibrioquimico 19661Document4 pagesArticulo Equilibrioquimico 19661dexgigiNo ratings yet
- Equilibrium Class XiDocument47 pagesEquilibrium Class XilololberuhlololNo ratings yet
- Kinetic Theory of GasesDocument9 pagesKinetic Theory of Gasestahasheikh822No ratings yet
- Gas Laws: M. L. WatsonDocument25 pagesGas Laws: M. L. WatsonAbhishek ChakrabartiNo ratings yet
- Equlibrium 2022-1Document15 pagesEqulibrium 2022-1Huzaifa Ahmed FarooqiNo ratings yet
- Ideal Gas LawDocument5 pagesIdeal Gas LawChristian Alic KelleyNo ratings yet
- Chemical EquilDocument11 pagesChemical EquilAditya VermaNo ratings yet
- Volume Additivity 1Document14 pagesVolume Additivity 1Kenneth Mendoza SorianoNo ratings yet
- Eso201A: Thermodynamics 2020-21 Ist Semester IIT Kanpur Instructor: P.A.ApteDocument16 pagesEso201A: Thermodynamics 2020-21 Ist Semester IIT Kanpur Instructor: P.A.ApteJitesh HemjiNo ratings yet
- Task 1 ST. ANISA 220105510003Document4 pagesTask 1 ST. ANISA 220105510003St. AnisaNo ratings yet
- YogeshDocument23 pagesYogeshAmandeep SinghNo ratings yet
- Chemical EquilibriumDocument36 pagesChemical EquilibriumBlitzkrieg AudiomachineNo ratings yet
- Chemical Equilibrium FDocument13 pagesChemical Equilibrium FRaju SinghNo ratings yet
- Chapter Glossary: Properties of Pure SubstancesDocument6 pagesChapter Glossary: Properties of Pure SubstancesyusufNo ratings yet
- Combustion EngineeringDocument196 pagesCombustion Engineeringdivakarshettyas4029100% (2)
- Chapter 4: Chemical EquilibriumDocument71 pagesChapter 4: Chemical EquilibriumYelbe FikruNo ratings yet
- Class 11 Chemistry Chapter 5 Study MaterialDocument33 pagesClass 11 Chemistry Chapter 5 Study MaterialmeghaNo ratings yet
- Equilibrium: Q Explain General Expression?Document7 pagesEquilibrium: Q Explain General Expression?Aq RaufNo ratings yet
- Anki How Fast Flash CardsDocument7 pagesAnki How Fast Flash CardsJ JokeNo ratings yet
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- DownloadDocument1 pageDownloadVIKRAM KUMARNo ratings yet
- This Study Resource Was: 5-1: Week 5 ExercisesDocument3 pagesThis Study Resource Was: 5-1: Week 5 ExercisesVIKRAM KUMARNo ratings yet
- A 35-kg Disk Rests On An Inclined Surface For Which Determine The Maximum Vertical Force P That May Be Applied To Link AB Without Causing The Disk To Slip at C. AnswerDocument1 pageA 35-kg Disk Rests On An Inclined Surface For Which Determine The Maximum Vertical Force P That May Be Applied To Link AB Without Causing The Disk To Slip at C. AnswerVIKRAM KUMARNo ratings yet
- 1 FP Per 162 Lines of C and 1 FP For 26 Lines of Smalltalk.Document2 pages1 FP Per 162 Lines of C and 1 FP For 26 Lines of Smalltalk.VIKRAM KUMARNo ratings yet
- Chap03 EX SolutionsDocument3 pagesChap03 EX SolutionsVIKRAM KUMARNo ratings yet
- 2,048 Block Frames Starting From B B 0 2,047 - So That R 11 Bits.Document4 pages2,048 Block Frames Starting From B B 0 2,047 - So That R 11 Bits.VIKRAM KUMARNo ratings yet
- Chapter 4 Exercise SolutionsDocument2 pagesChapter 4 Exercise SolutionsVIKRAM KUMARNo ratings yet
- Production Analysis and Compensation Policy: Questions and Answers Q7.1Document29 pagesProduction Analysis and Compensation Policy: Questions and Answers Q7.1VIKRAM KUMARNo ratings yet
- A File Filter Reads An Input FileDocument11 pagesA File Filter Reads An Input FileVIKRAM KUMARNo ratings yet
- The Clean Clothes Corner LaundryDocument3 pagesThe Clean Clothes Corner LaundryVIKRAM KUMARNo ratings yet
- Running Head: Questions Response 1Document3 pagesRunning Head: Questions Response 1VIKRAM KUMARNo ratings yet
- Carpet Size: Area Length×WidthDocument3 pagesCarpet Size: Area Length×WidthVIKRAM KUMARNo ratings yet
- 1 Final Strategic PlanDocument26 pages1 Final Strategic PlanVIKRAM KUMARNo ratings yet
- Artificial Intelligence (AI) and Neural NetworksDocument4 pagesArtificial Intelligence (AI) and Neural NetworksVIKRAM KUMARNo ratings yet
- Meeting ID Meeting Date Meeting Time Meeting Venue Event ID User ID Rating Person ID StatusDocument6 pagesMeeting ID Meeting Date Meeting Time Meeting Venue Event ID User ID Rating Person ID StatusVIKRAM KUMARNo ratings yet
- Computer Organisation Midterm - Ii Solution 12.30 PM To 2.30 PM Time: 2 HrsDocument4 pagesComputer Organisation Midterm - Ii Solution 12.30 PM To 2.30 PM Time: 2 HrsVIKRAM KUMARNo ratings yet
- AnswerDocument4 pagesAnswerVIKRAM KUMARNo ratings yet
- 1 TypicallyDocument4 pages1 TypicallyVIKRAM KUMAR0% (1)
- Auditing IP2Document2 pagesAuditing IP2VIKRAM KUMARNo ratings yet
- A Sample of 26 Offshore Oil Workers Took Part in A Simulated Escape ExerciseDocument1 pageA Sample of 26 Offshore Oil Workers Took Part in A Simulated Escape ExerciseVIKRAM KUMAR0% (1)
- A. List All The Primitive Data Types and Explain The Difference Between ThemDocument9 pagesA. List All The Primitive Data Types and Explain The Difference Between ThemVIKRAM KUMARNo ratings yet