Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
17 viewsMissen 12-5
Missen 12-5
Uploaded by
Rafi Bachtiar SaputraA first-order gas-phase decomposition reaction of compound A into compounds R and S is conducted in an adiabatic batch reactor. Initially, the reactor has a volume of 0.5 m3, temperature of 300 K, and pressure of 500 kPa. The reaction is first-order with respect to A with a rate constant of 1014e-10,000/T. Profiles of the fraction of A and temperature over time are determined, with the reaction considered complete when the fraction of A reaches 0.99.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
You might also like
- R134a SuperDocument6 pagesR134a Superprem singhNo ratings yet
- Odysseas Kopsidas - 9Document35 pagesOdysseas Kopsidas - 9Οδυσσεας ΚοψιδαςNo ratings yet
- Laboratorio Fisica III-Ley de OHMDocument10 pagesLaboratorio Fisica III-Ley de OHMLeandro RamirezNo ratings yet
- Odysseas Kopsidas - 8Document35 pagesOdysseas Kopsidas - 8Οδυσσεας ΚοψιδαςNo ratings yet
- Criterios de DiseñoDocument8 pagesCriterios de DiseñoKARITO SUAREZNo ratings yet
- Odysseas Kopsidas - 11Document35 pagesOdysseas Kopsidas - 11Οδυσσεας ΚοψιδαςNo ratings yet
- Odysseas Kopsidas - 7Document35 pagesOdysseas Kopsidas - 7Οδυσσεας ΚοψιδαςNo ratings yet
- App3Document2 pagesApp3hamidrezaee008No ratings yet
- Benceno-Etilbenceno RaoultDocument5 pagesBenceno-Etilbenceno RaoultGian GiancarlosNo ratings yet
- Destilacion BatchDocument116 pagesDestilacion BatchTanitDayanaPerezNo ratings yet
- Pengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)Document8 pagesPengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)SelametNo ratings yet
- Pengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)Document8 pagesPengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)SelametNo ratings yet
- Temperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Document8 pagesTemperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Melissa QuinteroNo ratings yet
- TF-8695 Therminol-66 Technical Bulletin-4Document1 pageTF-8695 Therminol-66 Technical Bulletin-4Graphios UtaNo ratings yet
- Practical Work N°4Document2 pagesPractical Work N°4Kaw TerNo ratings yet
- GRAFICADocument16 pagesGRAFICALENNI DAYARA HERNANDEZ GARCIANo ratings yet
- Calcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WaterDocument6 pagesCalcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WateraliNo ratings yet
- Rekapitulasi Uji Kesesuaian Distribusi Parameter StatistikDocument4 pagesRekapitulasi Uji Kesesuaian Distribusi Parameter StatistikAnin ArchNo ratings yet
- Estudio de Tanques Polar IDocument22 pagesEstudio de Tanques Polar ICristhian Huanqui TapiaNo ratings yet
- Practica MRUADocument3 pagesPractica MRUAMr. Hot RodNo ratings yet
- Vapor PressureDocument42 pagesVapor PressureSamuel OnyewuenyiNo ratings yet
- Diagram AsDocument11 pagesDiagram AsJhon MagañoNo ratings yet
- Analisis Benda TegarDocument2 pagesAnalisis Benda TegarTito HandikaNo ratings yet
- Fisicoquimica GraficasDocument2 pagesFisicoquimica GraficasPérez Carrasco Paulina GabrielaNo ratings yet
- Shank Area SpecificationsDocument3 pagesShank Area SpecificationsychodnekerNo ratings yet
- Solucionario - Tovar Soto RobinsonDocument11 pagesSolucionario - Tovar Soto RobinsonEnrique CarhuamacaNo ratings yet
- DataDocument3 pagesDatalaura sageNo ratings yet
- TrabajoDocument4 pagesTrabajoSofía RivasNo ratings yet
- Physical Properties of Gases and Liquids: AppendixDocument13 pagesPhysical Properties of Gases and Liquids: AppendixMarcos Vinicius KonopkaNo ratings yet
- Tablas Transferencia de MasaDocument25 pagesTablas Transferencia de MasaEdwin GuillénNo ratings yet
- Properties of Gases, Vapors, Liquids and Solids: Nitin GoelDocument12 pagesProperties of Gases, Vapors, Liquids and Solids: Nitin GoelSamir ZaghloolNo ratings yet
- Libro 1Document19 pagesLibro 1Quispe Mamani Yhesica NaydeNo ratings yet
- Perhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuDocument17 pagesPerhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuAis(y)ahNo ratings yet
- UUT Lab ReportDocument10 pagesUUT Lab ReportAyong AnisNo ratings yet
- Well Function W (U) - UDocument8 pagesWell Function W (U) - URendy Khoirul IlhamNo ratings yet
- Hydro Mechanical Erection Estimating 2021Document45 pagesHydro Mechanical Erection Estimating 2021samNo ratings yet
- Laboratorio 1Document7 pagesLaboratorio 1Karen RodriguezNo ratings yet
- Actividad3 2Document8 pagesActividad3 2Darleen Ariana Serruto AlarconNo ratings yet
- X Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaDocument4 pagesX Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaCarlos FloresNo ratings yet
- Pararel D - Sesi D2 - Grup M - Perhitungan VleDocument18 pagesPararel D - Sesi D2 - Grup M - Perhitungan VleNur RokhmaNo ratings yet
- Problem SolutionDocument4 pagesProblem SolutionKarar AL-DahlkiNo ratings yet
- Curva de RemanzoDocument15 pagesCurva de Remanzokebler kevNo ratings yet
- Hall EffectDocument9 pagesHall EffectThiberNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- NSSC Process Optimization: Ii. Spent Liquors: AbstractDocument8 pagesNSSC Process Optimization: Ii. Spent Liquors: AbstractKarteek KandalaNo ratings yet
- C1 C2 C3 C4 TC (K) TR: Feed Into ColumnDocument4 pagesC1 C2 C3 C4 TC (K) TR: Feed Into ColumnKarthik RajeshNo ratings yet
- Deck Slab DesignDocument18 pagesDeck Slab DesignNeelakandan PrakashNo ratings yet
- Odysseas Kopsidas - 12Document35 pagesOdysseas Kopsidas - 12Οδυσσεας ΚοψιδαςNo ratings yet
- Odysseas Kopsidas - 5Document35 pagesOdysseas Kopsidas - 5Οδυσσεας ΚοψιδαςNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- Solucion t2 - LoyolaDocument9 pagesSolucion t2 - LoyolaTamara Loyola LunaNo ratings yet
- Miguel Quispe MolloDocument5 pagesMiguel Quispe Mollomaruja olveaNo ratings yet
- MR U A Industrial 2019Document11 pagesMR U A Industrial 2019IvanNo ratings yet
- Protensão PerdasDocument8 pagesProtensão PerdasLucas GasperinNo ratings yet
- Protensão PerdasDocument8 pagesProtensão PerdasLucas GasperinNo ratings yet
- MWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterDocument2 pagesMWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterbastianpurwaNo ratings yet
- P10 LMF2Document7 pagesP10 LMF2Kevin Rivera GarciaNo ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- United States Census Figures Back to 1630From EverandUnited States Census Figures Back to 1630No ratings yet
- 1 s2.0 S1877705817350543 Main PDFDocument7 pages1 s2.0 S1877705817350543 Main PDFRafi Bachtiar SaputraNo ratings yet
- Skripsi Tanpa Bab PembahasanDocument73 pagesSkripsi Tanpa Bab PembahasanRafi Bachtiar SaputraNo ratings yet
- Available Online At: Basic TheoryDocument9 pagesAvailable Online At: Basic TheoryRafi Bachtiar SaputraNo ratings yet
- Available Online At: Procedia Engineering 44 (2012) 787 - 788Document2 pagesAvailable Online At: Procedia Engineering 44 (2012) 787 - 788Rafi Bachtiar SaputraNo ratings yet
Missen 12-5
Missen 12-5
Uploaded by
Rafi Bachtiar Saputra0 ratings0% found this document useful (0 votes)
17 views1 pageA first-order gas-phase decomposition reaction of compound A into compounds R and S is conducted in an adiabatic batch reactor. Initially, the reactor has a volume of 0.5 m3, temperature of 300 K, and pressure of 500 kPa. The reaction is first-order with respect to A with a rate constant of 1014e-10,000/T. Profiles of the fraction of A and temperature over time are determined, with the reaction considered complete when the fraction of A reaches 0.99.
Original Description:
soal missen 12-5
TRKI
Copyright
© © All Rights Reserved
Available Formats
XLSX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA first-order gas-phase decomposition reaction of compound A into compounds R and S is conducted in an adiabatic batch reactor. Initially, the reactor has a volume of 0.5 m3, temperature of 300 K, and pressure of 500 kPa. The reaction is first-order with respect to A with a rate constant of 1014e-10,000/T. Profiles of the fraction of A and temperature over time are determined, with the reaction considered complete when the fraction of A reaches 0.99.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
0 ratings0% found this document useful (0 votes)
17 views1 pageMissen 12-5
Missen 12-5
Uploaded by
Rafi Bachtiar SaputraA first-order gas-phase decomposition reaction of compound A into compounds R and S is conducted in an adiabatic batch reactor. Initially, the reactor has a volume of 0.5 m3, temperature of 300 K, and pressure of 500 kPa. The reaction is first-order with respect to A with a rate constant of 1014e-10,000/T. Profiles of the fraction of A and temperature over time are determined, with the reaction considered complete when the fraction of A reaches 0.99.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
You are on page 1of 1
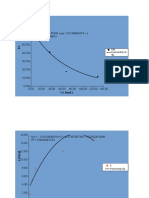
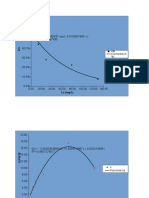
12-5 Missen
A gas-phase decomposition, A R + S, is to be conducted in a batch reactor, with initial
conditions of T0, = 300 K, V0 , = 0.5 m3, and a (constant) total pressure of 500 kPa. The values
of Cp , for A, R, and S are, respectively, 185.6, 104.7, and 80.9 J mol -1 K-1. The enthalpy of
reaction is -6280 J (mol A)-l, and the reaction is first-order with respect to A, with k A = 1014e
h . Determine the profiles of fA and T versus t, if the process is adiabatic, and T and t for
-10,000/T -l
fA = 0.99
Dik :
To 300 K Cpr 104.7 mol-1K-1
Vo 0.5 m3 Cps 80.9 mol-1K-1
P 500 kPa fa 0.99
Cpa 185.6 mol-1K-1 -6280 J (mol A)-1
ka 10^14e-1000h-1
Jawab
niCp 185.6 nAo
j fa T/K Ka/h-1 G G* t/h
1 0.00 300.0 0.334 3.00 0.00
2 0.10 303.4 0.484 2.30 2.65 0.25
3 0.20 306.8 0.696 1.80 2.05 0.44
4 0.30 310.1 0.993 1.44 1.62 0.59
5 0.40 313.5 1.405 1.19 1.31 0.72
6 0.50 316.9 1.975 1.01 1.10 0.82
7 0.60 320.3 2.755 0.91 0.96 0.92
8 0.70 323.7 3.817 0.87 0.89 1.00
9 0.80 327.0 5.253 0.95 0.91 1.10
10 0.90 330.4 7.183 1.39 1.17 1.23
11 0.99 333.5 9.467 10.56 5.98 1.97
You might also like
- R134a SuperDocument6 pagesR134a Superprem singhNo ratings yet
- Odysseas Kopsidas - 9Document35 pagesOdysseas Kopsidas - 9Οδυσσεας ΚοψιδαςNo ratings yet
- Laboratorio Fisica III-Ley de OHMDocument10 pagesLaboratorio Fisica III-Ley de OHMLeandro RamirezNo ratings yet
- Odysseas Kopsidas - 8Document35 pagesOdysseas Kopsidas - 8Οδυσσεας ΚοψιδαςNo ratings yet
- Criterios de DiseñoDocument8 pagesCriterios de DiseñoKARITO SUAREZNo ratings yet
- Odysseas Kopsidas - 11Document35 pagesOdysseas Kopsidas - 11Οδυσσεας ΚοψιδαςNo ratings yet
- Odysseas Kopsidas - 7Document35 pagesOdysseas Kopsidas - 7Οδυσσεας ΚοψιδαςNo ratings yet
- App3Document2 pagesApp3hamidrezaee008No ratings yet
- Benceno-Etilbenceno RaoultDocument5 pagesBenceno-Etilbenceno RaoultGian GiancarlosNo ratings yet
- Destilacion BatchDocument116 pagesDestilacion BatchTanitDayanaPerezNo ratings yet
- Pengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)Document8 pagesPengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)SelametNo ratings yet
- Pengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)Document8 pagesPengaruh Perubahan Konsentrasi Terhadap Nilai Esel (Teoritis)SelametNo ratings yet
- Temperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Document8 pagesTemperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Melissa QuinteroNo ratings yet
- TF-8695 Therminol-66 Technical Bulletin-4Document1 pageTF-8695 Therminol-66 Technical Bulletin-4Graphios UtaNo ratings yet
- Practical Work N°4Document2 pagesPractical Work N°4Kaw TerNo ratings yet
- GRAFICADocument16 pagesGRAFICALENNI DAYARA HERNANDEZ GARCIANo ratings yet
- Calcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WaterDocument6 pagesCalcium Chloride - English Units: Density, Composition and TCT of 94-97% Cacl Solutions in WateraliNo ratings yet
- Rekapitulasi Uji Kesesuaian Distribusi Parameter StatistikDocument4 pagesRekapitulasi Uji Kesesuaian Distribusi Parameter StatistikAnin ArchNo ratings yet
- Estudio de Tanques Polar IDocument22 pagesEstudio de Tanques Polar ICristhian Huanqui TapiaNo ratings yet
- Practica MRUADocument3 pagesPractica MRUAMr. Hot RodNo ratings yet
- Vapor PressureDocument42 pagesVapor PressureSamuel OnyewuenyiNo ratings yet
- Diagram AsDocument11 pagesDiagram AsJhon MagañoNo ratings yet
- Analisis Benda TegarDocument2 pagesAnalisis Benda TegarTito HandikaNo ratings yet
- Fisicoquimica GraficasDocument2 pagesFisicoquimica GraficasPérez Carrasco Paulina GabrielaNo ratings yet
- Shank Area SpecificationsDocument3 pagesShank Area SpecificationsychodnekerNo ratings yet
- Solucionario - Tovar Soto RobinsonDocument11 pagesSolucionario - Tovar Soto RobinsonEnrique CarhuamacaNo ratings yet
- DataDocument3 pagesDatalaura sageNo ratings yet
- TrabajoDocument4 pagesTrabajoSofía RivasNo ratings yet
- Physical Properties of Gases and Liquids: AppendixDocument13 pagesPhysical Properties of Gases and Liquids: AppendixMarcos Vinicius KonopkaNo ratings yet
- Tablas Transferencia de MasaDocument25 pagesTablas Transferencia de MasaEdwin GuillénNo ratings yet
- Properties of Gases, Vapors, Liquids and Solids: Nitin GoelDocument12 pagesProperties of Gases, Vapors, Liquids and Solids: Nitin GoelSamir ZaghloolNo ratings yet
- Libro 1Document19 pagesLibro 1Quispe Mamani Yhesica NaydeNo ratings yet
- Perhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuDocument17 pagesPerhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuAis(y)ahNo ratings yet
- UUT Lab ReportDocument10 pagesUUT Lab ReportAyong AnisNo ratings yet
- Well Function W (U) - UDocument8 pagesWell Function W (U) - URendy Khoirul IlhamNo ratings yet
- Hydro Mechanical Erection Estimating 2021Document45 pagesHydro Mechanical Erection Estimating 2021samNo ratings yet
- Laboratorio 1Document7 pagesLaboratorio 1Karen RodriguezNo ratings yet
- Actividad3 2Document8 pagesActividad3 2Darleen Ariana Serruto AlarconNo ratings yet
- X Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaDocument4 pagesX Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaCarlos FloresNo ratings yet
- Pararel D - Sesi D2 - Grup M - Perhitungan VleDocument18 pagesPararel D - Sesi D2 - Grup M - Perhitungan VleNur RokhmaNo ratings yet
- Problem SolutionDocument4 pagesProblem SolutionKarar AL-DahlkiNo ratings yet
- Curva de RemanzoDocument15 pagesCurva de Remanzokebler kevNo ratings yet
- Hall EffectDocument9 pagesHall EffectThiberNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- NSSC Process Optimization: Ii. Spent Liquors: AbstractDocument8 pagesNSSC Process Optimization: Ii. Spent Liquors: AbstractKarteek KandalaNo ratings yet
- C1 C2 C3 C4 TC (K) TR: Feed Into ColumnDocument4 pagesC1 C2 C3 C4 TC (K) TR: Feed Into ColumnKarthik RajeshNo ratings yet
- Deck Slab DesignDocument18 pagesDeck Slab DesignNeelakandan PrakashNo ratings yet
- Odysseas Kopsidas - 12Document35 pagesOdysseas Kopsidas - 12Οδυσσεας ΚοψιδαςNo ratings yet
- Odysseas Kopsidas - 5Document35 pagesOdysseas Kopsidas - 5Οδυσσεας ΚοψιδαςNo ratings yet
- Co (MG/L) : A% Exponential (A %)Document35 pagesCo (MG/L) : A% Exponential (A %)Οδυσσεας ΚοψιδαςNo ratings yet
- Solucion t2 - LoyolaDocument9 pagesSolucion t2 - LoyolaTamara Loyola LunaNo ratings yet
- Miguel Quispe MolloDocument5 pagesMiguel Quispe Mollomaruja olveaNo ratings yet
- MR U A Industrial 2019Document11 pagesMR U A Industrial 2019IvanNo ratings yet
- Protensão PerdasDocument8 pagesProtensão PerdasLucas GasperinNo ratings yet
- Protensão PerdasDocument8 pagesProtensão PerdasLucas GasperinNo ratings yet
- MWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterDocument2 pagesMWH S Water Treatment Principles and Design Third Edition - 2012 - Crittenden - Appendix C Physical Properties of WaterbastianpurwaNo ratings yet
- P10 LMF2Document7 pagesP10 LMF2Kevin Rivera GarciaNo ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- United States Census Figures Back to 1630From EverandUnited States Census Figures Back to 1630No ratings yet
- 1 s2.0 S1877705817350543 Main PDFDocument7 pages1 s2.0 S1877705817350543 Main PDFRafi Bachtiar SaputraNo ratings yet
- Skripsi Tanpa Bab PembahasanDocument73 pagesSkripsi Tanpa Bab PembahasanRafi Bachtiar SaputraNo ratings yet
- Available Online At: Basic TheoryDocument9 pagesAvailable Online At: Basic TheoryRafi Bachtiar SaputraNo ratings yet
- Available Online At: Procedia Engineering 44 (2012) 787 - 788Document2 pagesAvailable Online At: Procedia Engineering 44 (2012) 787 - 788Rafi Bachtiar SaputraNo ratings yet