Professional Documents
Culture Documents
Behaviour of Gases - PDF
Behaviour of Gases - PDF
Uploaded by
Nasih AhmadOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Behaviour of Gases - PDF
Behaviour of Gases - PDF
Uploaded by
Nasih AhmadCopyright:
Available Formats
Reservoir Fluid Properties
Behaviour of Gases:
A gas is a homogenous fluid that has no definite volume but fills completely the vessel in which
it is placed.
Ideal gas:
An ideal gas with the following assumptions:
• Volume of the molecules i.e. insignificant with respect to the total volume of the gas.
• There are no attractive or repulsive forces between molecules or between molecules and
container walls.
• There is no internal energy loss when molecules collide.
Out of these assumptions come the following equations.
Boyle’s Law
At constant temperature the volume of a given weight of a gas is inversely proportional to the
pressure of a gas.
V α 1/P or PV = constant, T is constant
Charles’ Law
At constant pressure, the volume of a given weight of gas varies directly with the temperature:
VαT or V/ T = constant, P is constant
The pressure and temperature in both laws are in absolute units.
Avogadro’s Law
Under the same conditions of temperature and pressure equal volumes of all ideal gases contain
the same number of molecules. That is, one molecular weight of any ideal gas occupies the same
volume as the molecular weight of another ideal gas at a given T and P.
Specifically, these are:
• 2.73 x 1026 molecules/lb mole of ideal gas.
• One molecular weight (in lbs) of any ideal gas at 60°F and 14.7 psia occupies a volume of
379.4 cu ft.
• One mole or gram-mol of a substance contains 6.02x1023 molecules and occupy at the
gas state a volume equal to:
- 22.414 l at 0°C and 1 atm
- 22.645 l at 15°C and 1 atm
- 23.694 l at 60°F and 1 atm
One mole of a material is a quantity of that material whose mass in the unit system selected is
numerically equal to the molecular weight.
One lb mole of methane CH4 = 16 lb
One kg mole of methane CH4 = 16kg
The Equation of State for an Ideal Gas
By combining the above laws an equation of state relating pressure, temperature and volume of
a gas is obtained.
Petroleum Engineering Dept. Page 1
Reservoir Fluid Properties
𝑃𝑉
= 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡
𝑇
R is the constant when the quantity of gas is equal to one mole. Its value depends on the unit
system used, so that;
R in oilfield units = 10.732 cu ft psia/ lb mole °R
Values of R for different unit systems
For n moles the equation becomes:
PV=nRT
T= absolute temperature oK or oR where ºK = 273 + oC and oR = 460 + oF
To find the volume occupied by a quantity of gas when the conditions of temperature and
pressure are changed from state 1 to state 2 we note that:
𝑃1 𝑉1 𝑃2 𝑉2
=
𝑇1 𝑇2
Exercise1
A gas cylinder contains methane at 1000 psia and 70°F. If the cylinder has a volume of 3 cu.ft
assuming methane is an ideal gas, calculate the mass of methane in the cylinder.
Solution:
P V = n R T, n = m/M
Where n = number of moles, m = mass, M = molecular weight
m = P M V/R T
Mass of methane, m = 8.46 lb
The Density of an Ideal Gas
Gas density, ρg = weight / volume = m / V
For 1 mole m = MW, MW = Molecular weight
Petroleum Engineering Dept. Page 2
Reservoir Fluid Properties
V = RT/P
MW . P
ρg =
RT
Exercise 2
Calculate the density of the gas in the cylinder in exercise 1
Solution:
Density of gas, ρg = 2.82 lb/cu ft
Standard Conditions
It is common practice to relate volumes oil and gas at reservoir conditions to conditions at
surface, i.e 14.7 psia and 60°F.
sc - standard conditions res - reservoir conditions
This relationship assumes that reservoir properties behave as ideal. This is NOT the case as will
be discussed later.
Exercise 3
Assuming methane is at the conditions of excercise 1, calculate the volume the gas would occupy
at standard conditions.
Solution:
Mixtures of Ideal Gases:
Dalton’s Law of Partial Pressures
Petroleum Engineering Dept. Page 3
Reservoir Fluid Properties
The total pressure exerted by a mixture of gases is equal to the sum of the pressures exerted by
its components. The partial pressure is the contribution to pressure of the individual component.
P = PA + PB + PC + …………… A, B and C are components, therefore:
where yj = mole fraction of jth component.
The partial pressure of a component, is the total pressure times the mole fraction.
Amagat’s Law
Amagat’s Law states that the volume occupied by an ideal gas mixture is equal to the sum of the
volumes that the pure components would occupy at the same temperature and pressure.
V = VA + VB + VC
For an ideal gas the volume fraction is equal to the mole fraction.
Exercise 4
A gas is made up of the following components; 25lb of methane, 3 lb of ethane and 1.5 lb of
propane. Express the composition of the gas in weight and mole fractions.
Solution:
Apparent Molecular Weight
Petroleum Engineering Dept. Page 4
Reservoir Fluid Properties
A mixture does not have a molecular weight although it behaves as though it had a molecular
weight. This is called the apparent molecular weight. AMW
AMW = Σ(Y j × MW j)
AMW for air = 28.97, a value of 29.0 is usually sufficiently accurate.
Exercise 5
What is the apparent molecular weight of the gas in exercise 4
Solution:
Specific Gravity of a Gas
The specific gravity of a gas, g is the ratio of the density of the gas relative to that of dry air at
the same conditions.
Assuming that the gases and air are ideal:
Mg = AMW of gas mixture, Mair = AMW of air.
Exercise 6
What is the gas gravity of the gas in exercise 4?
Solution:
BEHAVIOUR OF REAL GASES:
Petroleum Engineering Dept. Page 5
Reservoir Fluid Properties
There are two general methods of correcting the ideal gas law equation:
1. By using a correction factor in the equation P V = n RT
2. By using another equation-of-state
Compressibility Factor for Natural Gases
The correction factor ‘z’ which is a function of the gas composition, pressure and temperature is
used to modify the ideal gas law to:
PV=ZnRT
Z is the compressibility factor and the equation is the compressibility equation-of-state or the
compressibility equation. The compressibility factor is not a constant but varies with changes in
gas composition, temperature and pressure and must be determined experimentally.
To compare two states the law now takes the form:
Law of Corresponding States
The law of corresponding states shows that the properties of many pure liquids and gases have
the same value at the same reduced temperature (Tr) and pressure (Pr):
The Law can be applied to mixtures by defining parameters called pseudo critical temperature,
Tpc and pseudocritical pressure, Ppc
where y is the mole fraction of component j and T cj and Pcj are the critical temperature and
pressure of component j
Exercise 7
Calculate the pseudocritical temperature and pseudocritical pressure of the mixture in exercise 4
Solution:
Pseudocritical pressure = 668.4 psia
Pseudocritical temperature = 362 oR
Petroleum Engineering Dept. Page 6
Reservoir Fluid Properties
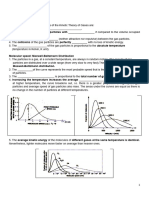
Compressibility factors for natural gas (Standing & Katz, Trans AIME, 1942)
Exercise 8
For the gas of exercise 4 determine the compressibility factor at a temperature of 150°F and a
pressure of 3500 psia
Solution:
Ppr = P/Ppc, Tpr = T / Tpc
From exercise 7 Ppc = 668 psia, Tpc = 362.6 oR
P = 3500 psia, and T = 150 oC i.e. 610 oR
Ppr = 5.24, and Tpr = 1.68
From Standing Katz chart
Compressibility factor, z = 0.88
Petroleum Engineering Dept. Page 7
You might also like
- 6th Central Pay Commission Salary CalculatorDocument15 pages6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- 1.reservoir Engineering Notes K PDFDocument116 pages1.reservoir Engineering Notes K PDFAzaru deen100% (1)
- GLWS9Document6 pagesGLWS9Vince HernándezNo ratings yet
- REN5415 Y19 Lec11&12Document69 pagesREN5415 Y19 Lec11&12Abdulla MohammadNo ratings yet
- Gas PropertiesDocument54 pagesGas PropertiesAli AhmedNo ratings yet
- Gas Behaviour EOSDocument59 pagesGas Behaviour EOSMurugavel ChandranNo ratings yet
- 5 - Behaviour of GasesDocument37 pages5 - Behaviour of Gasessiaskel100% (1)
- SC RE Chap5-GasesDocument49 pagesSC RE Chap5-Gasesweldsv0% (1)
- Non Reacting MixturesDocument6 pagesNon Reacting MixturesLalo RubioNo ratings yet
- Chapter 14Document42 pagesChapter 14Aubrey LanotNo ratings yet
- Chapter2 PGE381Document58 pagesChapter2 PGE381leeNo ratings yet
- Gas PropertiesDocument9 pagesGas PropertiesReza Gustarani DaneswariNo ratings yet
- CH 10 Gases StudentDocument48 pagesCH 10 Gases StudentTrọng NguyễnNo ratings yet
- Chapter 1 BasicsDocument35 pagesChapter 1 BasicsMohammed BahramNo ratings yet
- The Equation of State For A Perfect Gas: A. Boyle'S LawDocument9 pagesThe Equation of State For A Perfect Gas: A. Boyle'S LawCzarina Jane PeregrinNo ratings yet
- Ch01-Slides-1 Gas LawsDocument60 pagesCh01-Slides-1 Gas LawsDoreen BenezethNo ratings yet
- Gas PropertiesDocument10 pagesGas PropertiesNuri RuedaNo ratings yet
- Kmk20003 (Chapter 2) Week 3Document18 pagesKmk20003 (Chapter 2) Week 3mr bentoNo ratings yet
- IPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VDocument8 pagesIPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VRandy SooknananNo ratings yet
- Topic 1 - Gas Laws (Part 2)Document45 pagesTopic 1 - Gas Laws (Part 2)Joshua LaBordeNo ratings yet
- THE GASEOUS STATE Notes 20septDocument13 pagesTHE GASEOUS STATE Notes 20septP YNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- Lec 21 22 - CH 13Document21 pagesLec 21 22 - CH 13samhameed2No ratings yet
- Chapter 5 CHEM110Document59 pagesChapter 5 CHEM110gracetetu102No ratings yet
- The Equation-Of State of An Ideal Gas Is Found To BeDocument4 pagesThe Equation-Of State of An Ideal Gas Is Found To Beulol ululNo ratings yet
- Properties of Gases - 220308 - 154934Document28 pagesProperties of Gases - 220308 - 154934Dhruvi PadmaniNo ratings yet
- A. Ideal GasesDocument40 pagesA. Ideal GasesDanan GentleNo ratings yet
- Chapter 4 Single-Phase SystemDocument38 pagesChapter 4 Single-Phase SystemRenu SekaranNo ratings yet
- Behaviour of GasesDocument29 pagesBehaviour of GasesAli RazaNo ratings yet
- For 2nd Year CK&EC Chapter 4 Final PDFDocument51 pagesFor 2nd Year CK&EC Chapter 4 Final PDFbahru demekeNo ratings yet
- 10 Gases 2b PDFDocument10 pages10 Gases 2b PDFchewazableNo ratings yet
- LO 5.3-The Ideal Gas Law and Its Applications: Chapter 6.4 + 6.5 in The TextbookDocument38 pagesLO 5.3-The Ideal Gas Law and Its Applications: Chapter 6.4 + 6.5 in The TextbookAmina AlmarzooqiNo ratings yet
- 5.1 Pressure: Chapter 5: GasesDocument4 pages5.1 Pressure: Chapter 5: GasesSam ChungNo ratings yet
- Presentation1 EOSDocument18 pagesPresentation1 EOSFares TobasyNo ratings yet
- 3 Ideal Gas LawDocument24 pages3 Ideal Gas LawEMMANUEL DELOS SANTOSNo ratings yet
- Natural Gas Physical PropertiesDocument20 pagesNatural Gas Physical PropertiesMarco Antonio Pomahuali BravoNo ratings yet
- PVT (Properties of Petroleum Fluids)Document32 pagesPVT (Properties of Petroleum Fluids)Oscar Mauricio TellezNo ratings yet
- Lecture 4 Gas Laws and RelationsDocument28 pagesLecture 4 Gas Laws and RelationsArsal SohrabNo ratings yet
- Gases and Other Properties: Lesson 5Document7 pagesGases and Other Properties: Lesson 5lucifer angelNo ratings yet
- Physical Chemistry: Chemical EngineeringDocument11 pagesPhysical Chemistry: Chemical EngineeringEd Ryan RualesNo ratings yet
- Gases, Vapors, Liquids and Solids: Basic Principle II Second Class Dr. Arkan Jasim HadiDocument13 pagesGases, Vapors, Liquids and Solids: Basic Principle II Second Class Dr. Arkan Jasim Hadiالزهور لخدمات الانترنيتNo ratings yet
- 4.1 Ideal GasesDocument22 pages4.1 Ideal GasesAnonymous o97HYLpe0No ratings yet
- 2 - Properties of Pure SubstanceDocument39 pages2 - Properties of Pure Substancerashedramadan46No ratings yet
- Gas Properties I 2018Document55 pagesGas Properties I 2018Johny ImitazNo ratings yet
- 3.4 Ideal Gas LawDocument15 pages3.4 Ideal Gas LawfaridaisepicNo ratings yet
- PV RT: Equations of StateDocument11 pagesPV RT: Equations of StateJeff HardyNo ratings yet
- CHNG 2002 - Topic 1 - Volumetric Properties of FluidsDocument29 pagesCHNG 2002 - Topic 1 - Volumetric Properties of FluidsElisha DanielNo ratings yet
- 1 23 Gas Calculations PDFDocument6 pages1 23 Gas Calculations PDFschool adressNo ratings yet
- The Ideal - Gas Equation of StateDocument13 pagesThe Ideal - Gas Equation of StateAudu SanusiNo ratings yet
- Mechnotes: Unit - 1 ObjectiveDocument25 pagesMechnotes: Unit - 1 ObjectiveKaran SelvaNo ratings yet
- Topic 05 - States of Matter - TutorsDocument17 pagesTopic 05 - States of Matter - TutorsTran Nhat ThangNo ratings yet
- Intermolecular Forces, Liquids, and Solids: General ChemistryDocument82 pagesIntermolecular Forces, Liquids, and Solids: General ChemistryMinh Khoi Tran NguyenNo ratings yet
- Gas Mixtures Study Guide in Powerpoint: To AccompanyDocument21 pagesGas Mixtures Study Guide in Powerpoint: To AccompanyDon HoNo ratings yet
- Chem 181 Chemistry of GasesDocument15 pagesChem 181 Chemistry of GasesJoey PooleNo ratings yet
- Gases, Vapors, Liquids, and SolidsDocument9 pagesGases, Vapors, Liquids, and SolidsAwadhNo ratings yet
- 03 Ideal GasDocument29 pages03 Ideal GasAdhel EnjelNo ratings yet
- SCH 103 NotesDocument50 pagesSCH 103 NotesJacqueseNo ratings yet
- Chapter 5 GasesDocument100 pagesChapter 5 GasesFABIO DE LIMANo ratings yet
- Gas Laws / Gases BehaviourDocument35 pagesGas Laws / Gases Behaviour9338-Anmol KatharNo ratings yet
- Physical and Chemical Equilibrium for Chemical EngineersFrom EverandPhysical and Chemical Equilibrium for Chemical EngineersRating: 5 out of 5 stars5/5 (1)
- Predicting IPR - Standing MethodDocument36 pagesPredicting IPR - Standing MethodNasih AhmadNo ratings yet
- HW # 3 Decline Curve AnalysisDocument3 pagesHW # 3 Decline Curve AnalysisNasih AhmadNo ratings yet
- QUIZ 4thDocument1 pageQUIZ 4thNasih Ahmad100% (1)
- Plagiarism Scan Report: Plagiarised Unique Words CharactersDocument1 pagePlagiarism Scan Report: Plagiarised Unique Words CharactersNasih AhmadNo ratings yet
- University of Zakho: Collage of Engineering Petroleum DepartmentDocument2 pagesUniversity of Zakho: Collage of Engineering Petroleum DepartmentNasih AhmadNo ratings yet
- Partial Differential Equations Example Sheet 1: BooksDocument6 pagesPartial Differential Equations Example Sheet 1: BooksNasih AhmadNo ratings yet
- Final Exam, F.Att - Pet.Geo.2019-2020Document2 pagesFinal Exam, F.Att - Pet.Geo.2019-2020Nasih AhmadNo ratings yet
- The U.S. Oil Supply Revolution and The Global EconomyDocument33 pagesThe U.S. Oil Supply Revolution and The Global EconomyNasih AhmadNo ratings yet
- Reservoir Engineering 1 (Week 1 & 2)Document35 pagesReservoir Engineering 1 (Week 1 & 2)Nasih AhmadNo ratings yet
- Engineering Analysis: Faculty of Engineering Petroleum EngineeringDocument7 pagesEngineering Analysis: Faculty of Engineering Petroleum EngineeringNasih AhmadNo ratings yet
- Summary of Course Outline During This Two Day Seminar, You Will - .Document2 pagesSummary of Course Outline During This Two Day Seminar, You Will - .Nasih AhmadNo ratings yet
- Qatar Economy and Problems Against ItDocument9 pagesQatar Economy and Problems Against ItNasih AhmadNo ratings yet
- majeed project٢Document24 pagesmajeed project٢Nasih AhmadNo ratings yet
- 3Rd Edition Stratigraphic Traps of The Middle East GTW: Registration BrochureDocument6 pages3Rd Edition Stratigraphic Traps of The Middle East GTW: Registration BrochureNasih AhmadNo ratings yet
- 6 Lecture Oil TrapsDocument14 pages6 Lecture Oil TrapsNasih Ahmad100% (1)
- Where:: Water Density 1000 or 1.0 or 8.33ppg Final Density Original Mud Volume Water Volume Final VolumeDocument1 pageWhere:: Water Density 1000 or 1.0 or 8.33ppg Final Density Original Mud Volume Water Volume Final VolumeNasih AhmadNo ratings yet
- 2.pressure Volume Work, Reversible Work, Irreversible WorkDocument3 pages2.pressure Volume Work, Reversible Work, Irreversible WorkKABHISHKA BALAMURUGAN (RA2311043010117)No ratings yet
- Meassurement and InstrumentationDocument223 pagesMeassurement and InstrumentationManuel Adrian Vidal PenedoNo ratings yet
- HydrostaticsDocument7 pagesHydrostaticsDeepak RamtekeNo ratings yet
- Natural Gas Energy Measurement PDFDocument354 pagesNatural Gas Energy Measurement PDFHenry Maeda100% (3)
- Blowout Resistance of Room-Temperature Vulcanized ElastomersDocument7 pagesBlowout Resistance of Room-Temperature Vulcanized ElastomersAhmad Zubair RasulyNo ratings yet
- HAL-ZoneSeal Isolation ProcessDocument11 pagesHAL-ZoneSeal Isolation ProcesszbhdzpNo ratings yet
- Synthron Case Study Write UpDocument10 pagesSynthron Case Study Write UpTallo CruzNo ratings yet
- Rosemount Contacting-Conductivity-SensorsDocument16 pagesRosemount Contacting-Conductivity-SensorscolbyNo ratings yet
- Eng U2 Module 3 - PlumbingDocument27 pagesEng U2 Module 3 - PlumbingNovelyn Butih-lingNo ratings yet
- Grade 8 Class TestDocument3 pagesGrade 8 Class TestshamshadNo ratings yet
- Ijmet 10 01 008Document15 pagesIjmet 10 01 008IAEME PUBLICATIONNo ratings yet
- FluidMech - Fluid PropertiesDocument3 pagesFluidMech - Fluid Propertiesocabonita01No ratings yet
- Adder 1Document2 pagesAdder 1Macarena GuzmanNo ratings yet
- 2023-000092CMP - TechnicalDocument12 pages2023-000092CMP - Technicalinfo.infinitytechnicalNo ratings yet
- Experiment No.1 (Difussion) FINALDocument7 pagesExperiment No.1 (Difussion) FINALSharmaine RoseNo ratings yet
- FLU GSN 2021R1 EN WS06 Duct VanesDocument48 pagesFLU GSN 2021R1 EN WS06 Duct Vanes2nnnfhx4kqNo ratings yet
- 17421-2019-Winter-Question-Paper (Msbte Study Resources)Document4 pages17421-2019-Winter-Question-Paper (Msbte Study Resources)Pranay ManwarNo ratings yet
- Tech ExampleDocument59 pagesTech ExampleJurie_sk3608No ratings yet
- TEG Dehydration ProcessDocument20 pagesTEG Dehydration Processarraziy fauzanNo ratings yet
- Review of Literature On Steam AccumulatorDocument10 pagesReview of Literature On Steam Accumulatortamil vaananNo ratings yet
- Vapor Pressure and Boiling Point of Liquid MixtureDocument58 pagesVapor Pressure and Boiling Point of Liquid MixtureFrancis Philomenraj L RNo ratings yet
- Acoustic Determination of Producing and Static Bottomhole PressuresDocument40 pagesAcoustic Determination of Producing and Static Bottomhole PressuresVictor Hugo DiazNo ratings yet
- Thermofix LasinstructiesDocument174 pagesThermofix LasinstructiesDaniel GarcíaNo ratings yet
- 115Document19 pages115physicsdocs100% (1)
- IprDocument8 pagesIprCamilaNo ratings yet
- D04-V-E809 Instrument Air ReceiverDocument2 pagesD04-V-E809 Instrument Air Receiverpragnesh82011No ratings yet
- Gas Natural Transmision PDFDocument43 pagesGas Natural Transmision PDFRodrigo Vasquez GonzalesNo ratings yet
- Liquid Pumps R7 2Document24 pagesLiquid Pumps R7 2Santosh Singh100% (1)
- Agard CP 392Document228 pagesAgard CP 392Telemetro100% (2)