Professional Documents
Culture Documents
RESULT AND DISCUSSION Exp Soap
RESULT AND DISCUSSION Exp Soap
Uploaded by
nisasoberiCopyright:
Available Formats
You might also like
- Full Report UreaDocument103 pagesFull Report Ureanisasoberi100% (1)
- Lab Report Soap MakingDocument6 pagesLab Report Soap MakingAmihanNo ratings yet
- Saponification LabDocument3 pagesSaponification LabChelsea MontrichardNo ratings yet
- Lab Report DetergentDocument3 pagesLab Report DetergentqwertyfssNo ratings yet
- Detection of Carbon and HydrogenDocument9 pagesDetection of Carbon and HydrogenIvanne IdorotNo ratings yet
- Alcohols and PhenolsDocument8 pagesAlcohols and PhenolsMomer83% (6)
- Production of Toilet SoapDocument60 pagesProduction of Toilet SoapNana Kwame Boateng93% (46)
- Bar and Liquid Soap: Department of Chemical EngineeringDocument8 pagesBar and Liquid Soap: Department of Chemical EngineeringErnie Mark Patosa MaratasNo ratings yet
- Lab Scale Production of SoapDocument16 pagesLab Scale Production of SoapAshrul NasirNo ratings yet
- TOPIC 12 Soaps and DetergentsDocument14 pagesTOPIC 12 Soaps and DetergentsKaynine Kiko50% (2)
- Soap and Detergent ExperimentDocument12 pagesSoap and Detergent ExperimentAkmalhakim ZakariaNo ratings yet
- Soap Making and Fats TestingDocument94 pagesSoap Making and Fats TestingEra MelaniaNo ratings yet
- CHM144L Experiment 4Document2 pagesCHM144L Experiment 4zidrick benjamin100% (1)
- ShampoDocument7 pagesShampoMd. Badrul Islam0% (1)
- Lab Report Fabric SoftenerDocument3 pagesLab Report Fabric SoftenerqwertyfssNo ratings yet
- Soap and SaponificationDocument9 pagesSoap and SaponificationWanie Has100% (2)
- Principle of Molisch's TestDocument6 pagesPrinciple of Molisch's TestMg HNo ratings yet
- Lab 6 - Soap and DetergentDocument16 pagesLab 6 - Soap and DetergentamiraaikharahNo ratings yet
- Qualitative Tests For Fats and OilsDocument1 pageQualitative Tests For Fats and OilsMuhammad Aslam100% (1)
- Problem Set 1 Conc of SolutionDocument2 pagesProblem Set 1 Conc of SolutionB13 - Villanueva HubertNo ratings yet
- Discussion Saponification of SoapDocument3 pagesDiscussion Saponification of Soappijechad0% (1)
- Preparation of SoapDocument16 pagesPreparation of SoapNurr Hada25% (4)
- Group 3 - Laboratory Report 2 - Methane and Its PropertiesDocument22 pagesGroup 3 - Laboratory Report 2 - Methane and Its PropertiesJESSIE FREDRICK DALANIELNo ratings yet
- Literature Review PDFDocument6 pagesLiterature Review PDFfatinNo ratings yet
- Lab Report Exp 3 PDFDocument16 pagesLab Report Exp 3 PDFeizat abas100% (1)
- Major Ingredients Used in Cosmetic ProductsDocument57 pagesMajor Ingredients Used in Cosmetic ProductsZahra GhalamiNo ratings yet
- Ice Cream Lab ReportDocument1 pageIce Cream Lab Reportescuintla67% (3)
- Lab Report Dish Washing LiquidDocument9 pagesLab Report Dish Washing LiquidJanwel BristolNo ratings yet
- The Healing Nature of Mandala MakingDocument3 pagesThe Healing Nature of Mandala MakingNathaliaEicellRoseBuenoNo ratings yet
- Used Cooking Oil CandleDocument10 pagesUsed Cooking Oil Candlemohamad ashaziq75% (4)
- EXPERIMENT NO 2 Separation of An Organic Mixture, Re Crystallization and Melting Point DeterminationDocument7 pagesEXPERIMENT NO 2 Separation of An Organic Mixture, Re Crystallization and Melting Point DeterminationJanina NemisNo ratings yet
- LIPSTICKDocument59 pagesLIPSTICKLinhNguyeNo ratings yet
- Metal Filter in Purifying Used Cooking OilDocument17 pagesMetal Filter in Purifying Used Cooking Oilkgdelacruz24810No ratings yet
- Soap and DetergentDocument24 pagesSoap and DetergentCik Tiem Ngagiman80% (10)
- Formal ReportDocument4 pagesFormal ReportKatrina TaracatacNo ratings yet
- Saponification ProcessDocument4 pagesSaponification ProcessAddison JuttieNo ratings yet
- Analiticka Prasanja Od ScribdDocument37 pagesAnaliticka Prasanja Od ScribdDoe BlackNo ratings yet
- Candles Post Lab ReportDocument5 pagesCandles Post Lab ReportErwin Cabangal100% (1)
- Experiment 3-Sublimation and Melting Point DeterminationDocument3 pagesExperiment 3-Sublimation and Melting Point DeterminationEmilyn Millares100% (4)
- Lava LampDocument2 pagesLava LampHema Jothy0% (2)
- Polymers and PlasticsDocument3 pagesPolymers and PlasticsAnonymous cgKtuWzNo ratings yet
- CHE402 Mentoy 05Document6 pagesCHE402 Mentoy 05JAN JERICHO MENTOYNo ratings yet
- Sublimation and Melting Point DeterminationDocument3 pagesSublimation and Melting Point Determinationdevilyn101No ratings yet
- Chemical FuelsDocument62 pagesChemical FuelsKeshav Rai100% (2)
- Chapter One: Proposal On Soap Research ProjectDocument16 pagesChapter One: Proposal On Soap Research ProjectYonael MezmureNo ratings yet
- Lab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.Document16 pagesLab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.niniwani59No ratings yet
- Worksheet 4 Combustion of Gas and Liquid FuelDocument3 pagesWorksheet 4 Combustion of Gas and Liquid FuelLin Xian XingNo ratings yet
- GC2 - Chemical Equilibrium Part 1Document12 pagesGC2 - Chemical Equilibrium Part 1Ace LeycoNo ratings yet
- Final Report Experiment 7: Preparation and Characterization of HydrocarbonsDocument6 pagesFinal Report Experiment 7: Preparation and Characterization of HydrocarbonsJhei Mesina AfableNo ratings yet
- Lab 1Document2 pagesLab 1ayilna_suhaila100% (4)
- Preparation of Banana FlavorsDocument8 pagesPreparation of Banana Flavorsnathan3602No ratings yet
- Food Engineering Laboratory 2021Document2 pagesFood Engineering Laboratory 2021Kamran AlamNo ratings yet
- Soap Making PresentationDocument31 pagesSoap Making PresentationMonique PabloNo ratings yet
- MODULE 2 - Lesson 1 - Introduction To Lipids - Structure, Properties and Classification - Part 3Document73 pagesMODULE 2 - Lesson 1 - Introduction To Lipids - Structure, Properties and Classification - Part 3Louise AnneNo ratings yet
- What Happens During A Body Scrub?Document13 pagesWhat Happens During A Body Scrub?Mary Ann FrancisNo ratings yet
- Formulation and Evaluation of Liquid Soap Containing Herbal Antimicrobial AgentDocument7 pagesFormulation and Evaluation of Liquid Soap Containing Herbal Antimicrobial AgentxiuhtlaltzinNo ratings yet
- Lab Report SaponificationDocument5 pagesLab Report SaponificationMarcy NilNo ratings yet
- All About LeachingDocument3 pagesAll About Leachinganon_850753764No ratings yet
- OrganicDocument10 pagesOrganicreza rashidian malekiNo ratings yet
- Soap Is Produced by The Saponification (Hydrolysis) of A Triglyceride (Fat or Oil) - (See Figure 1.)Document5 pagesSoap Is Produced by The Saponification (Hydrolysis) of A Triglyceride (Fat or Oil) - (See Figure 1.)Vignesh SivakumarNo ratings yet
- Final Soap Production 1Document13 pagesFinal Soap Production 1SuvamNo ratings yet
- Chapter 1 Engineering Technology ProfessionDocument51 pagesChapter 1 Engineering Technology Professionnisasoberi100% (1)
- Graph SeperationDocument4 pagesGraph SeperationnisasoberiNo ratings yet
- GraphDocument10 pagesGraphnisasoberiNo ratings yet
- Concentration Naoh Vs Conductivity: 2.0 ResultsDocument6 pagesConcentration Naoh Vs Conductivity: 2.0 ResultsnisasoberiNo ratings yet
- Concentration Naoh Vs ConductivityDocument10 pagesConcentration Naoh Vs Conductivitynisasoberi0% (1)
- Pinned Plate and Finned Plate.: Air VelocityDocument1 pagePinned Plate and Finned Plate.: Air VelocitynisasoberiNo ratings yet
- RESULT N Discussion Exp 1Document6 pagesRESULT N Discussion Exp 1nisasoberiNo ratings yet
- Basic Quantities and SI Units. Physical QuantitiesDocument2 pagesBasic Quantities and SI Units. Physical QuantitiesnisasoberiNo ratings yet
- RESULT and DISCUSSIONDocument5 pagesRESULT and DISCUSSIONnisasoberiNo ratings yet
- Topic 5 Single Phase AC CircuitDocument51 pagesTopic 5 Single Phase AC Circuitnisasoberi100% (1)
- Parallel CircuitDocument9 pagesParallel CircuitnisasoberiNo ratings yet
- INTRODUCTIONDocument2 pagesINTRODUCTIONnisasoberiNo ratings yet
- Tutorial 5 Single Phase Three Phase With AnswerDocument2 pagesTutorial 5 Single Phase Three Phase With AnswernisasoberiNo ratings yet
- Tutorial 1 Basic Concept of Electrical With AnswerDocument3 pagesTutorial 1 Basic Concept of Electrical With AnswernisasoberiNo ratings yet
- Tutorial 5 Single Phase Three Phase With AnswerDocument2 pagesTutorial 5 Single Phase Three Phase With AnswernisasoberiNo ratings yet
- Tutorial 2 Cicuit Theorems With AnswerDocument9 pagesTutorial 2 Cicuit Theorems With AnswernisasoberiNo ratings yet
- Centrifugal CompressorDocument18 pagesCentrifugal CompressornisasoberiNo ratings yet
- Topic 1 Basic Concepts of ElectricityDocument20 pagesTopic 1 Basic Concepts of Electricitynisasoberi100% (2)
- Temperature (°C) VS Flow Rate (m3/hr) : FlowrateDocument14 pagesTemperature (°C) VS Flow Rate (m3/hr) : FlowratenisasoberiNo ratings yet
- English Activities Programme Minutes MeetingDocument6 pagesEnglish Activities Programme Minutes MeetingnisasoberiNo ratings yet
- Citation Civ192 /L 17417Document6 pagesCitation Civ192 /L 17417nisasoberiNo ratings yet
- NAME: - Id NoDocument2 pagesNAME: - Id NonisasoberiNo ratings yet
- CHAPTER 3 REFRIGERATION CYCLE (Complete Slide)Document19 pagesCHAPTER 3 REFRIGERATION CYCLE (Complete Slide)nisasoberiNo ratings yet
- CHAPTER 1 STEAM GENERATION (Complete Slide)Document25 pagesCHAPTER 1 STEAM GENERATION (Complete Slide)nisasoberiNo ratings yet
RESULT AND DISCUSSION Exp Soap
RESULT AND DISCUSSION Exp Soap
Uploaded by
nisasoberiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
RESULT AND DISCUSSION Exp Soap
RESULT AND DISCUSSION Exp Soap
Uploaded by
nisasoberiCopyright:
Available Formats
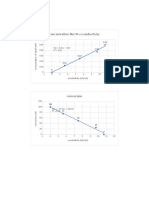
RESULT AND DISCUSSION
The object of the experiment was to use fatty acid from palm oil to manufacture the bar
Soap. As with all fats, palm oil is made up of fatty acids, esterified with glycerol. Palm oil has a
relatively high content of saturated fat, in particular the 16-carbon saturated fatty acid, palmitic
acid, which is named after it. Next is to assess the amount of soap produced in the experiment.
Soap is the concept used to evaluate the amount of soap generated in the experiment.
The experiment started when the oil was heated up to a Celsius of 70-80 degrees. Steam
coils heat up the mixture inside the kettle to get it to a boil. The mass thickens upon heating,
while the fat reacts to the alkali, creating soap and glycerin. Then, it divides normal fat into fatty
acids and glycerin. Afterwards, the fatty acids are distilled for purification. Next, 2 the purified
fatty acids are then mixed together with an accurate amount of alkali to form soap. Other
ingredients are also mixed in, such as abrasives and fragrances. Then, the hot liquid soap may be
whipped to add air. After that, the soap can be poured into molds, and can be hardened into a
large slab. This can also be refrigerated in a separate freezer. The slab is sliced into smaller bar-
size parts, which are then painted and sealed. The whole continuous cycle can be completed in
several hours, from breaking to finishing. Lastly, after a few hours of soap was withdrawn from
the ice box and heater and the soap compressor unit was used to create a formed soap.
Based on the result of this experiment, the soaps produced from this experiment are a bar-
shaped solid-liquid soap. Sodium hydroxide reacts with sodium chloride, EDTA and fatty oils
before the soap is produced. Next, the soap mixture was stirred at the temperature of 76.7
degrees Celsius. The response will not shift at first 10 minutes from the color of light yellow to
bright yellow. Within 30 minutes, it switches the phases to be absorbed and it reacts to solid-
liquid soap with fatty acids. There were a few possible errors in this experiment, which is that the
temperature may not have been set in the correct range, which may cause fatty oils not to react
with the mixture completely. Next, the time it took to wait for the reaction to occur may take a
long or short time until the reaction in this experiment does not occur and cannot fully react.
You might also like
- Full Report UreaDocument103 pagesFull Report Ureanisasoberi100% (1)
- Lab Report Soap MakingDocument6 pagesLab Report Soap MakingAmihanNo ratings yet
- Saponification LabDocument3 pagesSaponification LabChelsea MontrichardNo ratings yet
- Lab Report DetergentDocument3 pagesLab Report DetergentqwertyfssNo ratings yet
- Detection of Carbon and HydrogenDocument9 pagesDetection of Carbon and HydrogenIvanne IdorotNo ratings yet
- Alcohols and PhenolsDocument8 pagesAlcohols and PhenolsMomer83% (6)
- Production of Toilet SoapDocument60 pagesProduction of Toilet SoapNana Kwame Boateng93% (46)
- Bar and Liquid Soap: Department of Chemical EngineeringDocument8 pagesBar and Liquid Soap: Department of Chemical EngineeringErnie Mark Patosa MaratasNo ratings yet
- Lab Scale Production of SoapDocument16 pagesLab Scale Production of SoapAshrul NasirNo ratings yet
- TOPIC 12 Soaps and DetergentsDocument14 pagesTOPIC 12 Soaps and DetergentsKaynine Kiko50% (2)
- Soap and Detergent ExperimentDocument12 pagesSoap and Detergent ExperimentAkmalhakim ZakariaNo ratings yet
- Soap Making and Fats TestingDocument94 pagesSoap Making and Fats TestingEra MelaniaNo ratings yet
- CHM144L Experiment 4Document2 pagesCHM144L Experiment 4zidrick benjamin100% (1)
- ShampoDocument7 pagesShampoMd. Badrul Islam0% (1)
- Lab Report Fabric SoftenerDocument3 pagesLab Report Fabric SoftenerqwertyfssNo ratings yet
- Soap and SaponificationDocument9 pagesSoap and SaponificationWanie Has100% (2)
- Principle of Molisch's TestDocument6 pagesPrinciple of Molisch's TestMg HNo ratings yet
- Lab 6 - Soap and DetergentDocument16 pagesLab 6 - Soap and DetergentamiraaikharahNo ratings yet
- Qualitative Tests For Fats and OilsDocument1 pageQualitative Tests For Fats and OilsMuhammad Aslam100% (1)
- Problem Set 1 Conc of SolutionDocument2 pagesProblem Set 1 Conc of SolutionB13 - Villanueva HubertNo ratings yet
- Discussion Saponification of SoapDocument3 pagesDiscussion Saponification of Soappijechad0% (1)
- Preparation of SoapDocument16 pagesPreparation of SoapNurr Hada25% (4)
- Group 3 - Laboratory Report 2 - Methane and Its PropertiesDocument22 pagesGroup 3 - Laboratory Report 2 - Methane and Its PropertiesJESSIE FREDRICK DALANIELNo ratings yet
- Literature Review PDFDocument6 pagesLiterature Review PDFfatinNo ratings yet
- Lab Report Exp 3 PDFDocument16 pagesLab Report Exp 3 PDFeizat abas100% (1)
- Major Ingredients Used in Cosmetic ProductsDocument57 pagesMajor Ingredients Used in Cosmetic ProductsZahra GhalamiNo ratings yet
- Ice Cream Lab ReportDocument1 pageIce Cream Lab Reportescuintla67% (3)
- Lab Report Dish Washing LiquidDocument9 pagesLab Report Dish Washing LiquidJanwel BristolNo ratings yet
- The Healing Nature of Mandala MakingDocument3 pagesThe Healing Nature of Mandala MakingNathaliaEicellRoseBuenoNo ratings yet
- Used Cooking Oil CandleDocument10 pagesUsed Cooking Oil Candlemohamad ashaziq75% (4)
- EXPERIMENT NO 2 Separation of An Organic Mixture, Re Crystallization and Melting Point DeterminationDocument7 pagesEXPERIMENT NO 2 Separation of An Organic Mixture, Re Crystallization and Melting Point DeterminationJanina NemisNo ratings yet
- LIPSTICKDocument59 pagesLIPSTICKLinhNguyeNo ratings yet
- Metal Filter in Purifying Used Cooking OilDocument17 pagesMetal Filter in Purifying Used Cooking Oilkgdelacruz24810No ratings yet
- Soap and DetergentDocument24 pagesSoap and DetergentCik Tiem Ngagiman80% (10)
- Formal ReportDocument4 pagesFormal ReportKatrina TaracatacNo ratings yet
- Saponification ProcessDocument4 pagesSaponification ProcessAddison JuttieNo ratings yet
- Analiticka Prasanja Od ScribdDocument37 pagesAnaliticka Prasanja Od ScribdDoe BlackNo ratings yet
- Candles Post Lab ReportDocument5 pagesCandles Post Lab ReportErwin Cabangal100% (1)
- Experiment 3-Sublimation and Melting Point DeterminationDocument3 pagesExperiment 3-Sublimation and Melting Point DeterminationEmilyn Millares100% (4)
- Lava LampDocument2 pagesLava LampHema Jothy0% (2)
- Polymers and PlasticsDocument3 pagesPolymers and PlasticsAnonymous cgKtuWzNo ratings yet
- CHE402 Mentoy 05Document6 pagesCHE402 Mentoy 05JAN JERICHO MENTOYNo ratings yet
- Sublimation and Melting Point DeterminationDocument3 pagesSublimation and Melting Point Determinationdevilyn101No ratings yet
- Chemical FuelsDocument62 pagesChemical FuelsKeshav Rai100% (2)
- Chapter One: Proposal On Soap Research ProjectDocument16 pagesChapter One: Proposal On Soap Research ProjectYonael MezmureNo ratings yet
- Lab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.Document16 pagesLab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.niniwani59No ratings yet
- Worksheet 4 Combustion of Gas and Liquid FuelDocument3 pagesWorksheet 4 Combustion of Gas and Liquid FuelLin Xian XingNo ratings yet
- GC2 - Chemical Equilibrium Part 1Document12 pagesGC2 - Chemical Equilibrium Part 1Ace LeycoNo ratings yet
- Final Report Experiment 7: Preparation and Characterization of HydrocarbonsDocument6 pagesFinal Report Experiment 7: Preparation and Characterization of HydrocarbonsJhei Mesina AfableNo ratings yet
- Lab 1Document2 pagesLab 1ayilna_suhaila100% (4)
- Preparation of Banana FlavorsDocument8 pagesPreparation of Banana Flavorsnathan3602No ratings yet
- Food Engineering Laboratory 2021Document2 pagesFood Engineering Laboratory 2021Kamran AlamNo ratings yet
- Soap Making PresentationDocument31 pagesSoap Making PresentationMonique PabloNo ratings yet
- MODULE 2 - Lesson 1 - Introduction To Lipids - Structure, Properties and Classification - Part 3Document73 pagesMODULE 2 - Lesson 1 - Introduction To Lipids - Structure, Properties and Classification - Part 3Louise AnneNo ratings yet
- What Happens During A Body Scrub?Document13 pagesWhat Happens During A Body Scrub?Mary Ann FrancisNo ratings yet
- Formulation and Evaluation of Liquid Soap Containing Herbal Antimicrobial AgentDocument7 pagesFormulation and Evaluation of Liquid Soap Containing Herbal Antimicrobial AgentxiuhtlaltzinNo ratings yet
- Lab Report SaponificationDocument5 pagesLab Report SaponificationMarcy NilNo ratings yet
- All About LeachingDocument3 pagesAll About Leachinganon_850753764No ratings yet
- OrganicDocument10 pagesOrganicreza rashidian malekiNo ratings yet
- Soap Is Produced by The Saponification (Hydrolysis) of A Triglyceride (Fat or Oil) - (See Figure 1.)Document5 pagesSoap Is Produced by The Saponification (Hydrolysis) of A Triglyceride (Fat or Oil) - (See Figure 1.)Vignesh SivakumarNo ratings yet
- Final Soap Production 1Document13 pagesFinal Soap Production 1SuvamNo ratings yet
- Chapter 1 Engineering Technology ProfessionDocument51 pagesChapter 1 Engineering Technology Professionnisasoberi100% (1)
- Graph SeperationDocument4 pagesGraph SeperationnisasoberiNo ratings yet
- GraphDocument10 pagesGraphnisasoberiNo ratings yet
- Concentration Naoh Vs Conductivity: 2.0 ResultsDocument6 pagesConcentration Naoh Vs Conductivity: 2.0 ResultsnisasoberiNo ratings yet
- Concentration Naoh Vs ConductivityDocument10 pagesConcentration Naoh Vs Conductivitynisasoberi0% (1)
- Pinned Plate and Finned Plate.: Air VelocityDocument1 pagePinned Plate and Finned Plate.: Air VelocitynisasoberiNo ratings yet
- RESULT N Discussion Exp 1Document6 pagesRESULT N Discussion Exp 1nisasoberiNo ratings yet
- Basic Quantities and SI Units. Physical QuantitiesDocument2 pagesBasic Quantities and SI Units. Physical QuantitiesnisasoberiNo ratings yet
- RESULT and DISCUSSIONDocument5 pagesRESULT and DISCUSSIONnisasoberiNo ratings yet
- Topic 5 Single Phase AC CircuitDocument51 pagesTopic 5 Single Phase AC Circuitnisasoberi100% (1)
- Parallel CircuitDocument9 pagesParallel CircuitnisasoberiNo ratings yet
- INTRODUCTIONDocument2 pagesINTRODUCTIONnisasoberiNo ratings yet
- Tutorial 5 Single Phase Three Phase With AnswerDocument2 pagesTutorial 5 Single Phase Three Phase With AnswernisasoberiNo ratings yet
- Tutorial 1 Basic Concept of Electrical With AnswerDocument3 pagesTutorial 1 Basic Concept of Electrical With AnswernisasoberiNo ratings yet
- Tutorial 5 Single Phase Three Phase With AnswerDocument2 pagesTutorial 5 Single Phase Three Phase With AnswernisasoberiNo ratings yet
- Tutorial 2 Cicuit Theorems With AnswerDocument9 pagesTutorial 2 Cicuit Theorems With AnswernisasoberiNo ratings yet
- Centrifugal CompressorDocument18 pagesCentrifugal CompressornisasoberiNo ratings yet
- Topic 1 Basic Concepts of ElectricityDocument20 pagesTopic 1 Basic Concepts of Electricitynisasoberi100% (2)
- Temperature (°C) VS Flow Rate (m3/hr) : FlowrateDocument14 pagesTemperature (°C) VS Flow Rate (m3/hr) : FlowratenisasoberiNo ratings yet
- English Activities Programme Minutes MeetingDocument6 pagesEnglish Activities Programme Minutes MeetingnisasoberiNo ratings yet
- Citation Civ192 /L 17417Document6 pagesCitation Civ192 /L 17417nisasoberiNo ratings yet
- NAME: - Id NoDocument2 pagesNAME: - Id NonisasoberiNo ratings yet
- CHAPTER 3 REFRIGERATION CYCLE (Complete Slide)Document19 pagesCHAPTER 3 REFRIGERATION CYCLE (Complete Slide)nisasoberiNo ratings yet
- CHAPTER 1 STEAM GENERATION (Complete Slide)Document25 pagesCHAPTER 1 STEAM GENERATION (Complete Slide)nisasoberiNo ratings yet