Professional Documents
Culture Documents
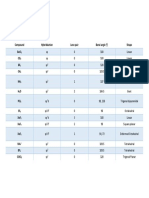
Block e Configuration Group (IUPAC) Group (CAS) : (n-2) F (n-1) D Ns
Block e Configuration Group (IUPAC) Group (CAS) : (n-2) F (n-1) D Ns
Uploaded by
Sakib KhanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Block e Configuration Group (IUPAC) Group (CAS) : (n-2) F (n-1) D Ns
Block e Configuration Group (IUPAC) Group (CAS) : (n-2) F (n-1) D Ns
Uploaded by
Sakib KhanCopyright:
Available Formats
Block e- configuration Group (IUPAC) Group (CAS)
s n s 1-2 1, 2, He I A, II A, VIII A
p n s 1-2 n p 1-6 13 - 18 III A - VIII A
d (n-1) d 1-10 n s 1-2 3 - 12 III B - VIII B, I B, II B
f (n-2) f 1–14 (n-1) d 0-1 ns 2 3 III B
Often you will be given the atomic numbers of elements (sometimes, just the names of
elements, if you are one with rotten luck), and be asked to position them in the periodic
table by their period and group number. Determining the period number is pretty
simple - you only need to figure out the number of shells in the atom. To find out the
group number, the following procedures should be used:
1. Determine the electronic structure of the atom and match it with one of the given
patterns in the table.
2. The group number is given by:
The number of electrons in the last s subshell for s block elements.
The number of electrons in the last s subshell plus the number of electrons in the
last p subshell for p block elements.
For d block elements, let n = number of electrons in d subshell + number of
electrons in s subshell.
If n ϵ { 3, 4, 5, 6, 7 }, groups are correspondingly III B - VII B.
If n ϵ { 8, 9, 10 }, the group number is VIII B.
If n ϵ { 11, 12 }, groups are correspondingly I B and II B.
Example: Fe (26) -> [Ar] 4s2 3p6 Group = 2 + 6 = 8 = VIII B
Example: Cu (29) -> [Ar] 4s1 3d10 Group = 1 + 10 = 11 = I B
N.B.: Please try a few of them from the periodic table yourselves. Let me know whether
you face issues.
You might also like
- Quantum Numbers WorksheetDocument2 pagesQuantum Numbers WorksheetCatalina PerryNo ratings yet
- Review Questions 4 PDFDocument6 pagesReview Questions 4 PDFUmme AbdullahNo ratings yet
- 3.1 Periodic TableDocument18 pages3.1 Periodic TablehaasNo ratings yet
- DPP-06 Nurture Periodic Table 1675162537243Document2 pagesDPP-06 Nurture Periodic Table 1675162537243Ayon BiswasNo ratings yet
- Work Sheet Atomic Structure, Periodic Table of The Elements & ClassesDocument3 pagesWork Sheet Atomic Structure, Periodic Table of The Elements & ClassessnezanaNo ratings yet
- Chapter 3.0: Periodic TableDocument3 pagesChapter 3.0: Periodic TablehernaniabdullahNo ratings yet
- Lec 3.1 - Classification of Elements SK015 PDFDocument24 pagesLec 3.1 - Classification of Elements SK015 PDFminaNo ratings yet
- 03 Periodic Properties Formula Sheets Getmarks AppDocument10 pages03 Periodic Properties Formula Sheets Getmarks Appmusk7597No ratings yet
- Fekok Uut Daeril: Cla-Lbl) VanDocument3 pagesFekok Uut Daeril: Cla-Lbl) VanChetan Surya SNo ratings yet
- DPP (For Vacation)Document2 pagesDPP (For Vacation)dharmesh kumarNo ratings yet
- Chapter 3 - Periodic TableDocument36 pagesChapter 3 - Periodic Tablekashvina paramjothyNo ratings yet
- FIS 0434 Chemistry 1 Assignment-Chapter 2Document1 pageFIS 0434 Chemistry 1 Assignment-Chapter 2Kim Seng OnnNo ratings yet
- AP Chemistry Quantum Numbers Worksheet F 2012Document5 pagesAP Chemistry Quantum Numbers Worksheet F 2012Aaronkim PalonNo ratings yet
- Homework Electron ConfigurationDocument3 pagesHomework Electron ConfigurationRoman AchkarNo ratings yet
- Periodic Table TheoryDocument24 pagesPeriodic Table TheorySatyam PandeyNo ratings yet
- SSLC Chemistry Chapter Wise Questions: Muhammed Muhsin CK 9207010369. 9946057991Document8 pagesSSLC Chemistry Chapter Wise Questions: Muhammed Muhsin CK 9207010369. 9946057991SajithKumarVariathNo ratings yet
- Sankalp Sheet - 6 Lectures - 8, 9 & 10: ATOMIC STRUCTURE Aufbau Principle, Pauli's Exclusion Principle, Hund's Rule, Electronic Configuration, NodesDocument2 pagesSankalp Sheet - 6 Lectures - 8, 9 & 10: ATOMIC STRUCTURE Aufbau Principle, Pauli's Exclusion Principle, Hund's Rule, Electronic Configuration, NodesGcgNo ratings yet
- Country's Best Online Test PlatformDocument9 pagesCountry's Best Online Test PlatformVardhan BunnuNo ratings yet
- DPP (31 To) IcDocument41 pagesDPP (31 To) IcRaju SinghNo ratings yet
- Work Sheet Atomic Structure, Periodic Table of The Elements & ClassesDocument2 pagesWork Sheet Atomic Structure, Periodic Table of The Elements & ClassessnezanaNo ratings yet
- Xi Chem Chapt3 PEriodic Properties of Elements WorksheetDocument10 pagesXi Chem Chapt3 PEriodic Properties of Elements WorksheetNandini Classes,City Light ,Surat. Cell (9429090525No ratings yet
- KleitmanRothschildSpencer76 - The Number of Semigroups of Order NDocument7 pagesKleitmanRothschildSpencer76 - The Number of Semigroups of Order NNOOB CS:GO PLAYERNo ratings yet
- Science8 Q3 Week6Document20 pagesScience8 Q3 Week6Kathrina De SenaNo ratings yet
- Periodic TableDocument23 pagesPeriodic TableHigh Tech FactsNo ratings yet
- ChemistryDocument2 pagesChemistryAnonymous 5eMcvrK1FBNo ratings yet
- 2Q ACTIVITY# 1 - Electronic Structure & PeriodicityDocument3 pages2Q ACTIVITY# 1 - Electronic Structure & PeriodicityAries Christian BaroyNo ratings yet
- CH - 4Document5 pagesCH - 4Phantom GamingNo ratings yet
- Periodic TableDocument4 pagesPeriodic TableMohit GargNo ratings yet
- Precal 2016 Special Exam PaperDocument3 pagesPrecal 2016 Special Exam PaperVipua KakeroNo ratings yet
- Periodic Table-1Document2 pagesPeriodic Table-1Gurmaan SinghNo ratings yet
- 3 - Classification of Elements - Work SheetDocument4 pages3 - Classification of Elements - Work SheetYogy YNo ratings yet
- Tenthclass-Newsyllabus-Studymaterial-Chemistryem-Classification - of - Elements - 9Document29 pagesTenthclass-Newsyllabus-Studymaterial-Chemistryem-Classification - of - Elements - 9ravitejakolaparthiNo ratings yet
- Test Bank For Chemistry 11th Edition ChangDocument18 pagesTest Bank For Chemistry 11th Edition Changa407347072No ratings yet
- Chemistry-08 11thDocument2 pagesChemistry-08 11thRaju SinghNo ratings yet
- Inorganic Chemistry by Team Neet SecretDocument152 pagesInorganic Chemistry by Team Neet Secret09 Krishna TrivediNo ratings yet
- CHP 3 Class 9Document1 pageCHP 3 Class 9Shah SaqibNo ratings yet
- Periodic Table WorksheetDocument23 pagesPeriodic Table Worksheetlakshmi ghayathri N.M.No ratings yet
- Genchem Act3Document4 pagesGenchem Act3YaniiNo ratings yet
- Test Bank For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFDocument36 pagesTest Bank For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFmarcus.saenz808100% (11)
- Chemistry 11th Edition by Chang ISBN 007766695X Test BankDocument20 pagesChemistry 11th Edition by Chang ISBN 007766695X Test Bankandrea100% (28)
- VK Jaiswal Problems in Inorganic Chemistry For JeeDocument538 pagesVK Jaiswal Problems in Inorganic Chemistry For JeeTanviNo ratings yet
- Atomic Structure L10 8 JULYDocument53 pagesAtomic Structure L10 8 JULYnavaneethj.surajNo ratings yet
- Electron Configuration 2Document6 pagesElectron Configuration 2268953No ratings yet
- Matter Bonding NoteDocument18 pagesMatter Bonding NotekasiwareeshaNo ratings yet
- Keam PaperDocument32 pagesKeam PaperMSbsuwbsqwNo ratings yet
- Quantum Number WorksheetDocument5 pagesQuantum Number Worksheetpatilsupriya930No ratings yet
- 2021 ClassificationDocument11 pages2021 ClassificationSora RoseNo ratings yet
- Chapter 8: Periodic Relationships Among The ElementsDocument14 pagesChapter 8: Periodic Relationships Among The Elements216435964No ratings yet
- Ts 8506 3. Periodic TableDocument7 pagesTs 8506 3. Periodic TableNURBALQIS BINTI ZAILAN BMNo ratings yet
- Class 10 2 SetsDocument8 pagesClass 10 2 SetsDEBASISH MOHANTYNo ratings yet
- Guided Revision Plan-Score AdvancedDocument5 pagesGuided Revision Plan-Score AdvancedPratham PatelNo ratings yet
- Periodic SystemDocument10 pagesPeriodic SystemAstuti GendaNo ratings yet
- Test Review For RetakeDocument3 pagesTest Review For RetakeVikramaadhithyaa JagannathanNo ratings yet
- XI Chem Unit - 3 (Combined)Document27 pagesXI Chem Unit - 3 (Combined)Kun HikaruNo ratings yet
- Clasification of Elements in The Periodic TableDocument81 pagesClasification of Elements in The Periodic TableAZIAH ABUNo ratings yet
- Soal Konfigurasi ElektronDocument3 pagesSoal Konfigurasi ElektronZuliJamiatiNo ratings yet
- Foundation Chemistry I - CHM 092 July - Nov 2020 Tutorial 4 (Topic 2)Document3 pagesFoundation Chemistry I - CHM 092 July - Nov 2020 Tutorial 4 (Topic 2)MUHAMMAD LUQMAN HAKIMI MOHD ZAMRINo ratings yet
- Structure of Atom Practice Questions and AnswersDocument30 pagesStructure of Atom Practice Questions and AnswersCheryl ChaudhariNo ratings yet
- Commensurabilities among Lattices in PU (1,n). (AM-132), Volume 132From EverandCommensurabilities among Lattices in PU (1,n). (AM-132), Volume 132No ratings yet
- Infrared Spectroscopy of Triatomics for Space ObservationFrom EverandInfrared Spectroscopy of Triatomics for Space ObservationNo ratings yet
- Compound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclDocument1 pageCompound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclSakib KhanNo ratings yet
- Chemistry-2 PDFDocument1 pageChemistry-2 PDFSakib KhanNo ratings yet
- Beginner #5 How Are You?: Lesson NotesDocument7 pagesBeginner #5 How Are You?: Lesson NotesSakib KhanNo ratings yet
- Beginner #3 What Is That?: Lesson NotesDocument5 pagesBeginner #3 What Is That?: Lesson NotesSakib KhanNo ratings yet
- Beginner #2 Where Are You From?: Lesson NotesDocument6 pagesBeginner #2 Where Are You From?: Lesson NotesSakib KhanNo ratings yet
- Beginner #1 What Is His Name?: Lesson NotesDocument6 pagesBeginner #1 What Is His Name?: Lesson NotesSakib KhanNo ratings yet
- English Medium Lec. Physics-2 2020-Ok PDFDocument23 pagesEnglish Medium Lec. Physics-2 2020-Ok PDFSakib KhanNo ratings yet