Professional Documents
Culture Documents
Pi Bonds and sp2 Hybridized Orbitals
Pi Bonds and sp2 Hybridized Orbitals
Uploaded by
Naveed Sajid0 ratings0% found this document useful (0 votes)
24 views3 pagesHybridization
Original Title
Pi bonds and sp2 hybridized orbitals
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentHybridization
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
24 views3 pagesPi Bonds and sp2 Hybridized Orbitals
Pi Bonds and sp2 Hybridized Orbitals
Uploaded by
Naveed SajidHybridization
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 3
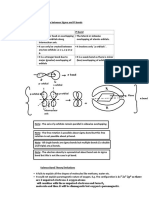
I touched on the idea of a sigma bond.
And that was a bond-- well, let me draw two nucleuses
and let me just draw one of the orbitals. Let's say this is an sp3 hybridized orbital, and that's
on this atom and this is kind of this big lobe right there. And then this guy has an sp3
hybridized orbital as well. That's the small lobe, and then that's the big lobe like that. A sigma
bond is one where there's an overlap kind of in the direction in which the lobes are pointed.
And you might say, well, how can there be any other type of bond than that? Well, the other
type of bond, so this right here-- let me make this clear. This right here is a sigma bond. And
you say, well, what other kind of bond could there be where my two orbitals overlap kind of
in the direction that they're pointing? And the other type of bond you could have, you can
imagine if you have two p orbitals. So let me draw the nucleus of two atoms, and I'll just
draw one of each of their p orbitals. So let's say that that's the nucleus and I'll just draw their
p orbitals. So a p orbital is just that dumbbell shape. Let me draw them a little bit closer
together. So a p orbital is that dumbbell shape. So let me draw this guy's-- one of his p
orbitals. I want to draw it a little bit bigger than that, and you'll see why a second. So one of
his p orbitals right there. It comes out like that. And then this guy over here also has a p
orbital that is parallel to this p orbital, so it goes like that. Let me draw that other one a little
bit straighter. It goes-- I want it to overlap more, so it goes like that. I think you get the idea.
So here, our two p orbitals are parallel to each other. This, you can imagine, these are sp3
hybridized orbitals. They're pointing at each other. Here, they're parallel. p orbitals are
parallel to each other, and you see that they overlap on this kind of top lobe here and in this
bottom lobe here. And this is a pi bond. Let me make this clear. And this is one pi bond. So
you could call it a pi, literally, with the Greek letter pi: pi bond. Sometimes you'll see this just
written as pi bond. And it's called a pi bond because it's the Greek letter for essentially p, and
we're dealing with p orbitals overlapping. Now sigma bonds, which are what form when you
have a single bond, these are stronger than pi bonds; pi bonds come into play once you start
forming double or triple bonds on top of a sigma bond. To kind of get a better visualization of
how that might work, let's think about ethene. So it's molecular structure looks like this. So
you have C double-bonded to C, and then each of those guys has two hydrogens. So let me
draw what it would look like, or our best visual, or our best ability to kind of conceptualize
what the orbitals around the carbon might look like. So first I'll draw the sp2 hybridized
orbitals. So let me just make it very clear what's going on here. So when we were dealing
with methane, which is just a carbon bonded to four hydrogens, and if I wanted to draw it in a
way that it kind of looks a little three-dimensional with a tetrahedral structure, it might look
like this. This hydrogen is pointing out a little bit. This hydrogen is kind of in the plane of the
page, and then maybe that hydrogen is behind it, and then you have one hydrogen popping
up. That's methane. And we saw that these were all sp3 hybridized orbitals around the
carbon, and then they each formed sigma bonds with each of the hydrogens. We saw that in
the last video. And when we drew its electron configuration, for this to happen, carbon's
electron configuration when bonding in methane needed to look like this. It needed to look
like 1s2. And then instead of having 2s2 and then 2p2, what you essentially have is-- let me
try it this way, actually, even better. Let me write this better. In 1s, you had two electrons,
and then instead of two s's, you had two electrons and on each of the p's, you had one, the s's
and the p's all got mixed up and you had a 2sp3 hybridized orbital, another 2sp3 hybridized
orbital, another 2sp3 hybridized orbital, and then another one, sp3. Normally, when carbon's
sitting by itself, you would expect a 2s here, and then you'd have a 2p in the x-direction, a 2p
in the y-direction, and then a 2p in the z-direction. But we saw in the last video, they all get
mixed up and they all have a 25% s-character, and a 75% p-character when carbon bonds in
methane and the electrons kind of separate out in that situation. When you're dealing with the
carbons in ethene, remember, eth- is for two carbons and ene-, because we're dealing with an
alkene. We have a double bond here. In this situation, the carbon's electron configuration
when they bond in ethene looks more like this. So you have your 1s, and the 1s orbital is still
full. It has two electrons in it. But then in your 2nd shell, I'll just write-- let me do this in a
different color. So in our 2nd shell, I'll show you what I mean in a second. I'm not writing the
s or p's so far on purpose, but we're going to have four electrons just like we had before.
We're still forming four bonds. We're going to have these four unpaired electrons. We're still
forming one, two, three, four bonds with each of the carbons, so they're going to be separated
out. But in this situation, instead of all of them being a mixture, kind of one part s, three parts
p, the s mixes with two of the p orbitals. So what you have is 2sp2 orbital. So you can
imagine that the s orbital mixes with two of the p orbitals. So now it's one part s, two parts p.
And then one of the p orbitals kind of stays by itself. And we need this p orbital to stay by
itself because it is going to be what's responsible for the pi bond. And we're going to see that
the pi bond does something very interesting to the molecule. It kind of makes it unrotatable
around a bond axis. And you'll see what I mean in a second. So let me see if I can, in three
dimensions, draw each of these carbons. So you have-- let me do it a different color. You
have this carbon right there. So let's say that's the nucleus. I'll put a C there so you know
which carbon we're dealing with. And then I'll draw-- you could assume that the 1s orbital,
it's really small right around the carbon. And then you have these hybridized orbitals, The
2sp2 orbitals, and they're all going to be planar, kind of forming a triangle, or I guess maybe a
peace sign on some level, but I'll try to draw it in three dimensions here. So you have one,
this is kind of coming out a little bit. Then you have one that's going in a little bit. And then
you have-- and they have another lobe a little bit on the other side, but I'm not going to draw
them. It'll complicate it. They still have characteristics of p, so they'll have two lobes, but one
is bigger than the other. And then you have one that's maybe going on this side. So you can
imagine that this is kind of a Mercedes sign if you drew a circle around it, on its side. So
that's this carbon right here. And, of course, it has its hydrogens. So you have this hydrogen
there. And so this hydrogen might be sitting right here. It just has one electron in its 1s
orbital. You have this hydrogen up here. It's sitting right over there. And now let's draw this
carbon. This carbon will be sitting-- I'm drawing it pretty close together. This carbon will be
sitting right there. He has his 1s orbital. They have the same electron configuration. He has
his 1s orbital right around him, and then he has the same configuration. Either of these guys,
we've so far only-- or in this first guy, I've only drawn these first three. I haven't drawn this
unhybridized p orbital yet. So I'll do that in a second. But let me draw his bonds. So first of
all, he has this, or you could imagine, that bond right there, which would be an sp2
hybridized bond. Let me do that in the same color. So he has this bond right here, which
would be an sp2 hybridized bond, just like that. And notice, this is a sigma bond. They
overlap in kind of the direction that they're pointing in. That's the best I could think about it.
And then he's got these two hydrogens, so one-- he's got this guy in the back, and then there's
one in the front. I'll draw it a little bigger so it's kind of pointing out at us, right? And then we
have this hydrogen is sitting right over here. And these are also sigma bonds, just to be very
clear about things. This is an s orbital overlapping with an sp2 orbital, but they're kind of
overlapping in the direction that they're pointed, or kind of along the direction of each other,
of the two atoms. This is a sigma bond, sigma bond, and then we have this hydrogen in the
back, which is also going to form a sigma bond. So everything I've drawn so far is a sigma
bond, so that, that. Maybe I don't want to make this picture too-- so I can just put sigma bond
there, sigma bond there, sigma bond there, sigma, sigma. So far I've drawn this bond, this
bond, this bond, this bond, and this bond, all of those sigma bonds. So, what happens to this
last p orbital for each of these guys? Well, that's going to be kind of sticking out of the plane
of the Mercedes sign, is the best way I can describe it. And let me see if I can do that in a
color that I haven't done yet. Oh, maybe this purple color. So you can imagine a pure p
orbital. So a pure p orbital, I'm going to need to draw it even bigger than that, actually. A
pure p orbital, it normally wouldn't be that big relative to things, but I have to make them
overlap. So it's a pure p orbital that's kind of going in, maybe you can imagine, the z-axis,
that the other orbitals are kind of a Mercedes sign in the x, y plane. And now you have the z-
axis going straight up and down, and those bottom two have to overlap so let me draw them
bigger. So it looks like that and it looks like that. And they're going straight up and down.
And notice, they are now overlapping. So this bond right here is this bond. I could've drawn
them in either way, but it's that second bond. And so what's happening now to the structure?
So let me make it very clear. This right here, that is a pi bond, and this right here is also-- it's
the same pi bond. It's this guy right here. It's the second bond in the double bond. But what's
happening here? Well, first of all, by itself it would be a weaker bond, but because we already
have a sigma bond that's making these molecules come closer together, this pi bond will
make them come even closer together. So this distance right here is closer than if we were to
just have a single sigma bond there. Now, on top of that, the really interesting thing is, if we
just had a sigma bond here, both of these molecules could kind of rotate around the bond
axis. They would be able to rotate around the bond axis if you just had one sigma bond there.
But since we have these pi bonds that are parallel to each other and they're kind of
overlapping and they're kind of locked in to that configuration, you can no longer rotate. If
one of these molecules rotates, the other one's going to rotate with it because these two guys
are locked together. So what this pi bond does in the situation is it makes this carbon-carbon
double bond-- it means that the double bonds are going to be rigid, that you can't have one
molecule kind of flipping, swapping these two hydrogens, without the other one having to
flip with it. So you wouldn't be able to kind of swap configurations of the hydrogens relative
to the other side. That's what it causes. So, hopefully, that gives you a good understanding of
the difference between sigma and pi bond. And if you're curious, when you're dealing with--
just to kind of make it clear, if we were dealing with ethyne, this is an example of ethene, but
ethyne looks like this. You have a triple bond. And so you have each side pointing to one
hydrogen. In this case, one of these, so the first bonds, you can imagine, so these bonds are
all sigma bonds. They're actually sp hybridized. Your 2s orbital only mixes with one of the
p's, so these are sp hybrid orbitals forming sigma bonds, so all of these right here. And then
both of these-- let me do this in different color. Both of these are pi bonds. And if you had to
imagine it, could imagine another pi bond kind of coming out of the page and another one
here coming out of the page and into the page, out and into the page, and they, too, are
overlapping, and you just have one hydrogen pointing out in each direction. Maybe I'll make
another video on that.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Test Bank For Organic Chemistry 7th Edition BruiceDocument20 pagesTest Bank For Organic Chemistry 7th Edition Bruicea157293279100% (2)
- Chapter 9 WorksheetsDocument18 pagesChapter 9 WorksheetsShaEshaa100% (1)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Resins For Surface Coatings Vol 2Document161 pagesResins For Surface Coatings Vol 2Lelecos100% (10)
- Semester 2 OrganicDocument1 pageSemester 2 OrganicNaveed SajidNo ratings yet
- Experiment No. 5 Preparation of Acetanilide Using Green Technique TheoryDocument2 pagesExperiment No. 5 Preparation of Acetanilide Using Green Technique TheoryNaveed SajidNo ratings yet
- Tests For Functional Groups: - Alkenes (C-C)Document2 pagesTests For Functional Groups: - Alkenes (C-C)Naveed SajidNo ratings yet
- CHT 314 Instrumental Methods of AnalysisDocument6 pagesCHT 314 Instrumental Methods of AnalysisNaveed SajidNo ratings yet
- Sample Transfer CertificateDocument1 pageSample Transfer CertificateNaveed SajidNo ratings yet
- Chapter No.1 Introduction of Instrumental Methods of AnalysisDocument1 pageChapter No.1 Introduction of Instrumental Methods of AnalysisNaveed SajidNo ratings yet
- For 2nd Chapter S. Q WavelengthDocument1 pageFor 2nd Chapter S. Q WavelengthNaveed SajidNo ratings yet
- 9: Multistep Synthesis (Experiment) : Step 1: Synthesis of BenzoinDocument4 pages9: Multistep Synthesis (Experiment) : Step 1: Synthesis of BenzoinNaveed SajidNo ratings yet
- 8: Identification of Unknowns (Experiment) : Solubility TestsDocument5 pages8: Identification of Unknowns (Experiment) : Solubility TestsNaveed SajidNo ratings yet
- 7: Distillation of A Mixture (Experiment) : ContributorsDocument1 page7: Distillation of A Mixture (Experiment) : ContributorsNaveed SajidNo ratings yet
- Organic PracticalDocument12 pagesOrganic PracticalNaveed SajidNo ratings yet
- 3: Extraction of Caffeine (Experiment) : ProcedureDocument1 page3: Extraction of Caffeine (Experiment) : ProcedureNaveed SajidNo ratings yet
- Hetero-Cyclic CompoundsDocument69 pagesHetero-Cyclic CompoundsNaveed SajidNo ratings yet
- Application Form For Bsc. Chemical Engineering: Sharif College of Engineering & TechnologyDocument2 pagesApplication Form For Bsc. Chemical Engineering: Sharif College of Engineering & TechnologyNaveed SajidNo ratings yet
- CVDocument21 pagesCVNaveed SajidNo ratings yet
- Contact Address: JOB RaiwandDocument2 pagesContact Address: JOB RaiwandNaveed SajidNo ratings yet
- 11th FineArts Model Paper PDFDocument4 pages11th FineArts Model Paper PDFNaveed SajidNo ratings yet
- Jan-E-Alam Executive Academy: AddressDocument2 pagesJan-E-Alam Executive Academy: AddressNaveed SajidNo ratings yet
- Chalcone MSDS: Section 1: Chemical Product and Company IdentificationDocument5 pagesChalcone MSDS: Section 1: Chemical Product and Company IdentificationNaveed SajidNo ratings yet
- ChemistryDocument48 pagesChemistryloretta00No ratings yet
- PGTRB Chemistry Unit 1 Study Materials English MediumDocument24 pagesPGTRB Chemistry Unit 1 Study Materials English MediumPAUL RAJNo ratings yet
- Lecture 1Document11 pagesLecture 1Fang GaoNo ratings yet
- Chemical Bonding PDFDocument86 pagesChemical Bonding PDFBlade RunnerNo ratings yet
- A New Look at The Chemical Bonding inDocument54 pagesA New Look at The Chemical Bonding inChandra Reddy100% (1)
- Huckel Molecular Orbital TheoryDocument4 pagesHuckel Molecular Orbital TheoryVijetha AchNo ratings yet
- Chemical Bonding ASSIGNMENTDocument4 pagesChemical Bonding ASSIGNMENTRiya Singh0% (1)
- PRINTED ChemT4HLQDocument18 pagesPRINTED ChemT4HLQtaengooNo ratings yet
- Chemical Bonding.4Document4 pagesChemical Bonding.4VIVEK RASTOGINo ratings yet
- Chem T4 HLQDocument17 pagesChem T4 HLQWilliam NguyenNo ratings yet
- Coordination Chemistry IIDocument77 pagesCoordination Chemistry IISOLeeNo ratings yet
- Chapter 1 Valence Bond TheoryDocument10 pagesChapter 1 Valence Bond Theoryaremyrah AzlanNo ratings yet
- Artistic EssayDocument6 pagesArtistic Essayafabkkkrb100% (1)
- 2009 A Levels P1 (No Worked Soln) and P2Document13 pages2009 A Levels P1 (No Worked Soln) and P2toh tim lamNo ratings yet
- Aroma Ti CityDocument33 pagesAroma Ti Citymidhungbabu88No ratings yet
- Chemical BondingDocument25 pagesChemical BondingpjaindakNo ratings yet
- Practicetopic 4 Paper 1Document10 pagesPracticetopic 4 Paper 1api-312595005No ratings yet
- CHEM14 - (5) The Chemical Bond 2Document81 pagesCHEM14 - (5) The Chemical Bond 2Kariza AbuNo ratings yet
- Lecture Notes Localized Delocalized Bonds Hyperconjugation Inductive Effect Charge TransferDocument5 pagesLecture Notes Localized Delocalized Bonds Hyperconjugation Inductive Effect Charge TransferBrian SamendeNo ratings yet
- VBTDocument29 pagesVBTsernaNo ratings yet
- AlkenesDocument12 pagesAlkenesDoc_CrocNo ratings yet
- Valence Bond Theory VBTDocument32 pagesValence Bond Theory VBTAsif AhnafNo ratings yet
- Unit 4 Chemical Bonding & Molecular StructureDocument29 pagesUnit 4 Chemical Bonding & Molecular StructureVighnesh0% (1)
- Bond and StructureDocument30 pagesBond and StructureRadu StafiNo ratings yet
- Aromatic Chemistry QuestionsDocument35 pagesAromatic Chemistry QuestionsBObNo ratings yet
- Answers To Eocqs: Cambridge International A Level ChemistryDocument2 pagesAnswers To Eocqs: Cambridge International A Level ChemistryCarissa Tabina RiandaNo ratings yet
- Chapter 7 - Structure and Synthesis of AlkenesDocument40 pagesChapter 7 - Structure and Synthesis of Alkenesrareair213No ratings yet
- 61 NotesDocument133 pages61 NotesEman NoamanNo ratings yet