Professional Documents
Culture Documents
19 - 7-PDF - Wiley S Problems in Organic Chemistry (With Solutions) For IIT JEE Main and Advanced Dr. K Singh
19 - 7-PDF - Wiley S Problems in Organic Chemistry (With Solutions) For IIT JEE Main and Advanced Dr. K Singh
Uploaded by
PranayOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
19 - 7-PDF - Wiley S Problems in Organic Chemistry (With Solutions) For IIT JEE Main and Advanced Dr. K Singh
19 - 7-PDF - Wiley S Problems in Organic Chemistry (With Solutions) For IIT JEE Main and Advanced Dr. K Singh
Uploaded by
PranayCopyright:
Available Formats
1.
1 HYBRIDIZATION, MOLECULAR FORMULA AND PHYSICAL PROPERTIES 3
P1.9 Identify the number of lone pairs of electrons that are
present in the following structures.
(a) (b)
P1.10 In the structures of Compounds (A), (B) and (C) given
below, identify (B)
(a) The molecular formulae.
(b) The number of sp2 and sp3 carbon atoms present.
(c) The number of lone pairs of electrons present.
P1.12 The following compound shows presence of two
nitrogen atoms. In this structure, identify
(A)
(B)
(a) The nature of orbital in which the lone pair on each N
atom reside.
(b) The hybridization of each N atom in the compound.
P1.13 In the following compounds:
(a) State the hybridization of the N atom?
(C)
(b) Which of these compounds is the most basic?
(a) The molecular formula for each compound.
P1.14 In the structure of acetonitrile given below
(b) Number of lone pairs of electrons present in each compound.
P1.11 Compounds (A) and (B) have the following struc-
(a) What is the hybridization of both the C atoms and N atom?
tures. In both the compounds, identify
(b) In what type of orbital does the lone pair on N reside?
(c) Identify the type of orbitals used to form each bond.
P1.15 In the structure of two naturally occurring compounds
given, identify the orbitals used to form the bonds
indicated with the arrows.
(A)
Chapter 1 Part I.indd 3 8/17/2015 11:03:36 AM
You might also like
- 16 7 PDF 8536693 Elliott Wave UnveiledDocument1 page16 7 PDF 8536693 Elliott Wave UnveiledPranayNo ratings yet
- Renk Type EM Install. & Oper.Document69 pagesRenk Type EM Install. & Oper.Don Freeman100% (5)
- Notes On Lipids Mam BarramedaDocument16 pagesNotes On Lipids Mam Barramedasshh bartolata100% (1)
- Supp Ex On Past Paper Atomic World I (Up To Bonding) (QN)Document2 pagesSupp Ex On Past Paper Atomic World I (Up To Bonding) (QN)England NgNo ratings yet
- Chemical Bond (SPM Q)Document11 pagesChemical Bond (SPM Q)Luna LatisyaNo ratings yet
- K.C.S.E Past Papers Questions Form 21Document29 pagesK.C.S.E Past Papers Questions Form 21Mutula NashonNo ratings yet
- ???? ?? ???????? ???????Document8 pages???? ?? ???????? ???????chopramanya34No ratings yet
- CMT 1302 Fundamentals of Chemistry For TechnologyDocument6 pagesCMT 1302 Fundamentals of Chemistry For TechnologyasdqweNo ratings yet
- Chemical Bonding Ex-4Document1 pageChemical Bonding Ex-4ansariarsalan1118aNo ratings yet
- Chapter 2Document3 pagesChapter 2MUHAMMAD LUQMAN HAKIMI MOHD ZAMRINo ratings yet
- Onesky,,,, Chem Form TwoDocument2 pagesOnesky,,,, Chem Form TwoOnesmusNo ratings yet
- General Chemistry II 102 PtsDocument10 pagesGeneral Chemistry II 102 PtsXyleen GregolaNo ratings yet
- Science Worksheet Solved Carbon and Its CompoundsDocument14 pagesScience Worksheet Solved Carbon and Its CompoundsKhuanna KapoorNo ratings yet
- ACFrOgDx1bVYvYs4ZSOHncy Lxwh252QwZ3fFL9jXIkG3a3C1VLDY Vr37kfXVajSVfx5aaqhaxlzrLaHGCZJqMVo6sl xJ6PpIbb 9PdW2nmPH61nrs58pY8k6KWgfbxmkEfVWkqRByaPp7m4RDocument9 pagesACFrOgDx1bVYvYs4ZSOHncy Lxwh252QwZ3fFL9jXIkG3a3C1VLDY Vr37kfXVajSVfx5aaqhaxlzrLaHGCZJqMVo6sl xJ6PpIbb 9PdW2nmPH61nrs58pY8k6KWgfbxmkEfVWkqRByaPp7m4RDr. Fatima IhsanNo ratings yet
- Atomic Structure & The Periodic Table 3 QPDocument9 pagesAtomic Structure & The Periodic Table 3 QPMagd OsamaNo ratings yet
- Chemistry 100Document9 pagesChemistry 100khushiii117777No ratings yet
- Microscopic World I PastpaperDocument3 pagesMicroscopic World I PastpaperCherry YamNo ratings yet
- Igcse Group 7 Elements 1 Ab Edit CompletedDocument1 pageIgcse Group 7 Elements 1 Ab Edit CompletedAvin AgarwalNo ratings yet
- Structure 1.1, 1.2, 1.3 PracticeDocument6 pagesStructure 1.1, 1.2, 1.3 PracticeEthan ElliotNo ratings yet
- Chemical Bonding SolutionsDocument44 pagesChemical Bonding SolutionsfbbNo ratings yet
- Genchem Tamu II (102 Items)Document10 pagesGenchem Tamu II (102 Items)Mark Ryan TripoleNo ratings yet
- Which Statement About An Atom Is True?: 1 Compiled by S Z Bangash Saint Mary' Academy Lalazar RWPDocument13 pagesWhich Statement About An Atom Is True?: 1 Compiled by S Z Bangash Saint Mary' Academy Lalazar RWPHamza KhalidNo ratings yet
- 1.10203 Tutorial 1Document2 pages1.10203 Tutorial 1McAdam TULAPINo ratings yet
- Topic 2 QuestionsDocument3 pagesTopic 2 QuestionsconcessiozacharyNo ratings yet
- F2 Chem TQDocument60 pagesF2 Chem TQsafinamuslimwomengroup.cboNo ratings yet
- BiologyDocument9 pagesBiologySharajNo ratings yet
- PS9001-CA Quiz AY 2015/16Document4 pagesPS9001-CA Quiz AY 2015/16Matthias Ecclesiastes TanNo ratings yet
- 3.3__atomic_structure_and_the_periodic_table_qp_-_igcse_cie_chemistry_-_extende_theory_paperDocument10 pages3.3__atomic_structure_and_the_periodic_table_qp_-_igcse_cie_chemistry_-_extende_theory_paperSamson YauNo ratings yet
- CHMS3Y20E2Document13 pagesCHMS3Y20E2no nameNo ratings yet
- Chemical Sciences Paper Ii: Meo MeoDocument13 pagesChemical Sciences Paper Ii: Meo MeoAmbarish MajiNo ratings yet
- SECTION A (15 Marks) Answer ALL Questions in This SectionDocument15 pagesSECTION A (15 Marks) Answer ALL Questions in This SectionFazliawati MahayuddinNo ratings yet
- Question Bank Atomic Structure: An Atom Uncharged ?Document13 pagesQuestion Bank Atomic Structure: An Atom Uncharged ?Tanay GuptaNo ratings yet
- Chemical Bonding Ex-1Document1 pageChemical Bonding Ex-1ansariarsalan1118aNo ratings yet
- Part 2 Microscopic World (I) LQDocument14 pagesPart 2 Microscopic World (I) LQWing LamNo ratings yet
- Adobe Scan 20-Jul-2023Document15 pagesAdobe Scan 20-Jul-2023Unknown UserNo ratings yet
- Chemical Bonding and Molecular Structure - DPPsDocument12 pagesChemical Bonding and Molecular Structure - DPPsRaxit PathakNo ratings yet
- Particles IIIDocument17 pagesParticles IIIhandryoutlookNo ratings yet
- Structure of AtomDocument1 pageStructure of AtomAman ChauhanNo ratings yet
- Science 2022 - RemovedDocument5 pagesScience 2022 - RemovedNithyaNo ratings yet
- Unit 2 Summative: Ap Chemistry Test BookletDocument6 pagesUnit 2 Summative: Ap Chemistry Test Bookletdanielyskim1119No ratings yet
- 5 D 9 DHSR U2 WCCfyp OXDDFDocument5 pages5 D 9 DHSR U2 WCCfyp OXDDFAradhana GuptaNo ratings yet
- MULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionDocument4 pagesMULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionElsie VanpraetNo ratings yet
- KeyDocument5 pagesKeyKali corgiNo ratings yet
- CHE 1010 Tutorial Sheet 3Document5 pagesCHE 1010 Tutorial Sheet 3Chimuka Onson MapikiNo ratings yet
- Chapter 16 (Wade)Document2 pagesChapter 16 (Wade)Mau BaraquelNo ratings yet
- Ach 110 AssignmentDocument8 pagesAch 110 AssignmentJoseph NyabugaNo ratings yet
- GR 10 Term 2 2018 Ps Worksheet Booklet PDFDocument44 pagesGR 10 Term 2 2018 Ps Worksheet Booklet PDFLucia ZeteleeNo ratings yet
- AL Chemistry 1996 Paper 1+2Document12 pagesAL Chemistry 1996 Paper 1+2api-3734333No ratings yet
- Error Less-12 ClassDocument62 pagesError Less-12 Classavantikarai1609No ratings yet
- Chapter 1.1 - Atoms and MoleculesDocument4 pagesChapter 1.1 - Atoms and MoleculesPAKK20622P Syarifah Nor Izzah binti Syed Abd HamidNo ratings yet
- Ch5 Quiz AnswersDocument4 pagesCh5 Quiz AnswersTony 852No ratings yet
- ExerciseDocument51 pagesExerciseyay100% (1)
- Work Sheet Chapter 1 Organic Chemistry IDocument4 pagesWork Sheet Chapter 1 Organic Chemistry IElfi Susanti VHNo ratings yet
- HL Bonding Revision QuestionsDocument9 pagesHL Bonding Revision QuestionsMrunal JadhavNo ratings yet
- Bonding Short TestDocument1 pageBonding Short TestAroon SoojaniNo ratings yet
- 07-Nuclear Physics IB ReviewDocument12 pages07-Nuclear Physics IB ReviewOnur YavuzcetinNo ratings yet
- Class 10 Science (02.02.2022)Document2 pagesClass 10 Science (02.02.2022)BHAVJOT KAUR SETHINo ratings yet
- 1.2 Atomic Structure (F) QPDocument6 pages1.2 Atomic Structure (F) QPMunachi UdengwuNo ratings yet
- Test 1Document2 pagesTest 1Windellea WongNo ratings yet
- 1st and 2nd Ionization EnergyDocument8 pages1st and 2nd Ionization Energy불곰국손가락 곰사냥꾼No ratings yet
- F.3 Chemistry Exercise 4 (Isotopes) (Q&A)Document4 pagesF.3 Chemistry Exercise 4 (Isotopes) (Q&A)SimonNo ratings yet
- Past Year Tutorial 2Document3 pagesPast Year Tutorial 2Fatin SyamimiNo ratings yet
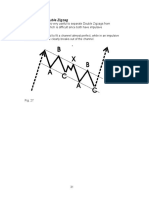
- Patterns: Classic Patterns vs. Modern Elliott Wave PatternsDocument1 pagePatterns: Classic Patterns vs. Modern Elliott Wave PatternsPranayNo ratings yet
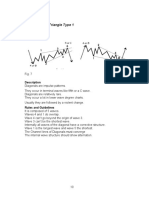
- Trends C. Diagonal Triangle Type 1: PatternDocument1 pageTrends C. Diagonal Triangle Type 1: PatternPranayNo ratings yet
- 1 - 7 PDF - 8536693 Elliott Wave UnveiledDocument1 page1 - 7 PDF - 8536693 Elliott Wave UnveiledPranayNo ratings yet
- 49 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page49 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- See The Successful Impulse Wave 1 On The AOL ChartDocument1 pageSee The Successful Impulse Wave 1 On The AOL ChartPranayNo ratings yet
- 31 - 7 PDF - 8536693 Elliott Wave UnveiledDocument1 page31 - 7 PDF - 8536693 Elliott Wave UnveiledPranayNo ratings yet
- 37 - 7 PDF - 8536693 Elliott Wave UnveiledDocument1 page37 - 7 PDF - 8536693 Elliott Wave UnveiledPranayNo ratings yet
- 17: History of World War I: by Albert E. Mckinley, PH.DDocument1 page17: History of World War I: by Albert E. Mckinley, PH.DPranayNo ratings yet
- 34 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page34 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- 31 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page31 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- 40 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David Butler PDFDocument1 page40 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David Butler PDFPranayNo ratings yet
- 37 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page37 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- 19 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page19 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 25 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page25 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- 13 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page13 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- 16 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page16 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- Preface To The Third Edition List of Abbreviations: Chapter 1. IntroductionDocument1 pagePreface To The Third Edition List of Abbreviations: Chapter 1. IntroductionPranayNo ratings yet
- 19 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerDocument1 page19 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David ButlerPranayNo ratings yet
- Phrase-Reading: Reading, Let Me Give You The Short Version of This TechniqueDocument1 pagePhrase-Reading: Reading, Let Me Give You The Short Version of This TechniquePranayNo ratings yet
- 25 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page25 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 22 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page22 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 31 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page31 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 7 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David Butler PDFDocument1 page7 - 7 PDF - 432352921 100 One Minute Speed Reading Drills by David Butler PDFPranayNo ratings yet
- 1 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page1 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranay67% (3)
- 49 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page49 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 28 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page28 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 16 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page16 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 43 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page43 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- 13 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersDocument1 page13 - 7-PDF - Wiley CandleStick and Pivot Point Trading TriggersPranayNo ratings yet
- Fatigue Analysis Using CAESAR IIDocument7 pagesFatigue Analysis Using CAESAR IIayounga100% (1)
- 01162018134131ferro Alloys 2016 (AdvanceRelease)Document25 pages01162018134131ferro Alloys 2016 (AdvanceRelease)Arjun KoduriNo ratings yet
- Addition Reactions of KetenesDocument27 pagesAddition Reactions of Ketenes張君睿No ratings yet
- Yio Chu Kang Secondary Sec 1 SA2 2020 ScienceDocument21 pagesYio Chu Kang Secondary Sec 1 SA2 2020 ScienceBecky ChungNo ratings yet
- Ich Q3BDocument31 pagesIch Q3BHussein Abu GhazalehNo ratings yet
- CGMP Regulations of Sterile ProductsDocument33 pagesCGMP Regulations of Sterile ProductsSukesh Potla75% (4)
- Sykam Tech SpecsDocument1 pageSykam Tech SpecsKremlin ReyesNo ratings yet
- HKALE Cytology (Enzyme) Question Paper and MSDocument20 pagesHKALE Cytology (Enzyme) Question Paper and MSNeetenin NeeteninNo ratings yet
- SECTION 03 30 00 Cast-In-Place ConcreteDocument36 pagesSECTION 03 30 00 Cast-In-Place ConcreteJuanPaoloYbañezNo ratings yet
- Chemical TestsDocument6 pagesChemical TestsAshwin UpretiNo ratings yet
- NBSS Final PresentationDocument15 pagesNBSS Final PresentationOmar Abd ElsalamNo ratings yet
- FM Global Property Loss Prevention Data SheetsDocument19 pagesFM Global Property Loss Prevention Data SheetsSuciu CatalinNo ratings yet
- Introduction To MicrobiologyDocument5 pagesIntroduction To MicrobiologyChristina EscardaNo ratings yet
- Waterbar Application ManualDocument6 pagesWaterbar Application ManualbarouniamineNo ratings yet
- Periodic Table and Chemical BondingDocument23 pagesPeriodic Table and Chemical BondingQSQF100% (1)
- Section 4. Metal Repair ProceduresDocument42 pagesSection 4. Metal Repair ProceduresrobinyNo ratings yet
- The Role of Collagen in Bone StrengthDocument19 pagesThe Role of Collagen in Bone StrengthClaudia AndreeaNo ratings yet
- Leister - Plastic-Welding - BR - Flooring - Interior-Decoration - USDocument24 pagesLeister - Plastic-Welding - BR - Flooring - Interior-Decoration - USNaufal IhsanNo ratings yet
- Astm E260-1996Document17 pagesAstm E260-1996Neo_SvaniNo ratings yet
- Style 1101 Rubber Expansion Joint: Shown With Control Unit GR/B Section DetailDocument1 pageStyle 1101 Rubber Expansion Joint: Shown With Control Unit GR/B Section DetailABDERRAZZAKNo ratings yet
- Soluforce High Temperature Pipes Productsheet PDFDocument2 pagesSoluforce High Temperature Pipes Productsheet PDFchrisgamellaNo ratings yet
- Aspirin Uv VisDocument6 pagesAspirin Uv VisseanNo ratings yet
- Soal KimfisDocument25 pagesSoal KimfisbagasNo ratings yet
- Comparison of Rutting Resistance of Stone Mastic Asphalt With Convention MixDocument7 pagesComparison of Rutting Resistance of Stone Mastic Asphalt With Convention MixUIJRT United International Journal for Research & TechnologyNo ratings yet
- Strengthening of RC Beams in Flexure Using Ferrocement: S.U. Khan, S.F.A.Rafeeqi and T. AyubDocument13 pagesStrengthening of RC Beams in Flexure Using Ferrocement: S.U. Khan, S.F.A.Rafeeqi and T. Ayubthomas13711363No ratings yet
- List of Materials For House Keeping: (To Be Submitted Along With Part-2 Price Bid)Document2 pagesList of Materials For House Keeping: (To Be Submitted Along With Part-2 Price Bid)Reninio100% (2)
- According To DIN 50150: Hardness Comparison TableDocument1 pageAccording To DIN 50150: Hardness Comparison Tablerbagri100% (1)
- Optimization of Fatigue Life of Welded Joints by Vikrant Ullhas Garud.Document89 pagesOptimization of Fatigue Life of Welded Joints by Vikrant Ullhas Garud.vikrant GarudNo ratings yet