Professional Documents
Culture Documents
9.2: The Solid State of Matter: Skills To Develop
9.2: The Solid State of Matter: Skills To Develop
Uploaded by
Graviton Manzano OlarteOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
9.2: The Solid State of Matter: Skills To Develop
9.2: The Solid State of Matter: Skills To Develop
Uploaded by
Graviton Manzano OlarteCopyright:
Available Formats
9.
2: The Solid State of Matter
Skills to Develop
Define and describe the bonding and properties of ionic, molecular, metallic, and covalent network crystalline
solids
Describe the main types of crystalline solids: ionic solids, metallic solids, covalent network solids, and molecular
solids
Explain the ways in which crystal defects can occur in a solid
When most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or
molecules are arranged in a definite repeating pattern. It is also possible for a liquid to freeze before its molecules become
arranged in an orderly pattern. The resulting materials are called amorphous solids or noncrystalline solids (or, sometimes,
glasses). The particles of such solids lack an ordered internal structure and are randomly arranged (Figure 9.2.1).
Figure 9.2.1 : The entities of a solid phase may be arranged in a regular, repeating pattern (crystalline solids) or
randomly (amorphous).
Metals and ionic compounds typically form ordered, crystalline solids. Substances that consist of large molecules, or a
mixture of molecules whose movements are more restricted, often form amorphous solids. For examples, candle waxes
are amorphous solids composed of large hydrocarbon molecules. Some substances, such as boron oxide (Figure 9.2.2),
can form either crystalline or amorphous solids, depending on the conditions under which it is produced. Also, amorphous
solids may undergo a transition to the crystalline state under appropriate conditions.
Figure 9.2.2 : (a) Diboron trioxide, B2O3, is normally found as a white, amorphous solid (a glass), which has a high
degree of disorder in its structure. (b) By careful, extended heating, it can be converted into a crystalline form of B2O3,
which has a very ordered arrangement.
Crystalline solids are generally classified according the nature of the forces that hold its particles together. These forces
are primarily responsible for the physical properties exhibited by the bulk solids. The following sections provide
descriptions of the major types of crystalline solids: ionic, metallic, covalent network, and molecular.
6/1/2020 9.2.1 CC-BY https://chem.libretexts.org/link?119768
Ionic Solids
Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together
by electrostatic attractions, which can be quite strong (Figure 9.2.3). Many ionic crystals also have high melting points.
This is due to the very strong attractions between the ions—in ionic compounds, the attractions between full charges are
(much) larger than those between the partial charges in polar molecular compounds. This will be looked at in more detail
in a later discussion of lattice energies. Although they are hard, they also tend to be brittle, and they shatter rather than
bend. Ionic solids do not conduct electricity; however, they do conduct when molten or dissolved because their ions are
free to move. Many simple compounds formed by the reaction of a metallic element with a nonmetallic element are ionic.
Figure 9.2.3 : Sodium chloride is an ionic solid.
Metallic Solids
Metallic solids such as crystals of copper, aluminum, and iron are formed by metal atoms Figure 9.2.4. The structure of
metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea” of delocalized electrons. The
atoms within such a metallic solid are held together by a unique force known as metallic bonding that gives rise to many
useful and varied bulk properties. All exhibit high thermal and electrical conductivity, metallic luster, and malleability.
Many are very hard and quite strong. Because of their malleability (the ability to deform under pressure or hammering),
they do not shatter and, therefore, make useful construction materials. The melting points of the metals vary widely.
Mercury is a liquid at room temperature, and the alkali metals melt below 200 °C. Several post-transition metals also have
low melting points, whereas the transition metals melt at temperatures above 1000 °C. These differences reflect
differences in strengths of metallic bonding among the metals.
Figure 9.2.4 : Copper is a metallic solid.
Covalent Network Solids
Covalent network solids include crystals of diamond, silicon, some other nonmetals, and some covalent compounds such
as silicon dioxide (sand) and silicon carbide (carborundum, the abrasive on sandpaper). Many minerals have networks of
covalent bonds. The atoms in these solids are held together by a network of covalent bonds, as shown in Figure 9.2.5. To
break or to melt a covalent network solid, covalent bonds must be broken. Because covalent bonds are relatively strong,
covalent network solids are typically characterized by hardness, strength, and high melting points. For example, diamond
is one of the hardest substances known and melts above 3500 °C.
6/1/2020 9.2.2 CC-BY https://chem.libretexts.org/link?119768
Figure 9.2.5 . A covalent crystal contains a three-dimensional network of covalent bonds, as illustrated by the structures
of diamond, silicon dioxide, silicon carbide, and graphite. Graphite is an exceptional example, composed of planar sheets
of covalent crystals that are held together in layers by noncovalent forces. Unlike typical covalent solids, graphite is very
soft and electrically conductive.
Molecular Solids
Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in Figure 9.2.6, are composed of neutral
molecules. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by
the melting points of the crystals. Small symmetrical molecules (nonpolar molecules), such as H2, N2, O2, and F2, have
weak attractive forces and form molecular solids with very low melting points (below −200 °C). Substances consisting of
larger, nonpolar molecules have larger attractive forces and melt at higher temperatures. Molecular solids composed of
molecules with permanent dipole moments (polar molecules) melt at still higher temperatures. Examples include ice
(melting point, 0 °C) and table sugar (melting point, 185 °C).
Figure 9.2.6 : Carbon dioxide (CO2) consists of small, nonpolar molecules and forms a molecular solid with a melting
point of −78 °C. Iodine (I2) consists of larger, nonpolar molecules and forms a molecular solid that melts at 114 °C.
Properties of Solids
A crystalline solid, like those listed in Table 9.2.1 has a precise melting temperature because each atom or molecule of the
same type is held in place with the same forces or energy. Thus, the attractions between the units that make up the crystal
all have the same strength and all require the same amount of energy to be broken. The gradual softening of an amorphous
material differs dramatically from the distinct melting of a crystalline solid. This results from the structural
nonequivalence of the molecules in the amorphous solid. Some forces are weaker than others, and when an amorphous
material is heated, the weakest intermolecular attractions break first. As the temperature is increased further, the stronger
attractions are broken. Thus amorphous materials soften over a range of temperatures.
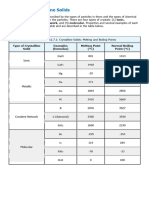
Table 9.2.1: Types of Crystalline Solids and Their Properties
Type of Solid Type of Particles Type of Attractions Properties Examples
6/1/2020 9.2.3 CC-BY https://chem.libretexts.org/link?119768
Type of Solid Type of Particles Type of Attractions Properties Examples
hard, brittle, conducts
electricity as a liquid but
ionic ions ionic bonds NaCl, Al2O3
not as a solid, high to very
high melting points
shiny, malleable, ductile,
conducts heat and
atoms of electropositive
metallic metallic bonds electricity well, variable Cu, Fe, Ti, Pb, U
elements
hardness and melting
temperature
atoms of electronegative very hard, not conductive,
covalent network covalent bonds C (diamond), SiO2, SiC
elements very high melting points
variable hardness, variable
brittleness, not
molecular molecules (or atoms) IMFs H2O, CO2, I2, C12H22O11
conductive, low melting
points
Graphene: Material of the Future
Carbon is an essential element in our world. The unique properties of carbon atoms allow the existence of carbon-
based life forms such as ourselves. Carbon forms a huge variety of substances that we use on a daily basis, including
those shown in Figure 9.2.7. You may be familiar with diamond and graphite, the two most common allotropes of
carbon. (Allotropes are different structural forms of the same element.) Diamond is one of the hardest-known
substances, whereas graphite is soft enough to be used as pencil lead. These very different properties stem from the
different arrangements of the carbon atoms in the different allotropes.
Figure 9.2.7 : Diamond is extremely hard because of the strong bonding between carbon atoms in all directions.
Graphite (in pencil lead) rubs off onto paper due to the weak attractions between the carbon layers. An image of a
graphite surface shows the distance between the centers of adjacent carbon atoms. (credit left photo: modification of
work by Steve Jurvetson; credit middle photo: modification of work by United States Geological Survey)
You may be less familiar with a recently discovered form of carbon: graphene. Graphene was first isolated in 2004 by
using tape to peel off thinner and thinner layers from graphite. It is essentially a single sheet (one atom thick) of
graphite. Graphene, illustrated in Figure 9.2.8, is not only strong and lightweight, but it is also an excellent conductor
of electricity and heat. These properties may prove very useful in a wide range of applications, such as vastly improved
computer chips and circuits, better batteries and solar cells, and stronger and lighter structural materials. The 2010
Nobel Prize in Physics was awarded to Andre Geim and Konstantin Novoselov for their pioneering work with
graphene.
6/1/2020 9.2.4 CC-BY https://chem.libretexts.org/link?119768
Figure 9.2.8 : Graphene sheets can be formed into buckyballs, nanotubes, and stacked layers.
Crystal Defects
In a crystalline solid, the atoms, ions, or molecules are arranged in a definite repeating pattern, but occasional defects may
occur in the pattern. Several types of defects are known, as illustrated in Figure 9.2.9. Vacancies are defects that occur
when positions that should contain atoms or ions are vacant. Less commonly, some atoms or ions in a crystal may occupy
positions, called interstitial sites, located between the regular positions for atoms. Other distortions are found in impure
crystals, as, for example, when the cations, anions, or molecules of the impurity are too large to fit into the regular
positions without distorting the structure. Trace amounts of impurities are sometimes added to a crystal (a process known
as doping) in order to create defects in the structure that yield desirable changes in its properties. For example, silicon
crystals are doped with varying amounts of different elements to yield suitable electrical properties for their use in the
manufacture of semiconductors and computer chips.
Figure 9.2.9 : Types of crystal defects include vacancies, interstitial atoms, and substitutions impurities.
Summary
6/1/2020 9.2.5 CC-BY https://chem.libretexts.org/link?119768
Video 9.2.1 : An overview of solids.
Some substances form crystalline solids consisting of particles in a very organized structure; others form amorphous
(noncrystalline) solids with an internal structure that is not ordered. The main types of crystalline solids are ionic solids,
metallic solids, covalent network solids, and molecular solids. The properties of the different kinds of crystalline solids are
due to the types of particles of which they consist, the arrangements of the particles, and the strengths of the attractions
between them. Because their particles experience identical attractions, crystalline solids have distinct melting
temperatures; the particles in amorphous solids experience a range of interactions, so they soften gradually and melt over
a range of temperatures. Some crystalline solids have defects in the definite repeating pattern of their particles. These
defects (which include vacancies, atoms or ions not in the regular positions, and impurities) change physical properties
such as electrical conductivity, which is exploited in the silicon crystals used to manufacture computer chips.
Glossary
amorphous solid
(also, noncrystalline solid) solid in which the particles lack an ordered internal structure
covalent network solid
solid whose particles are held together by covalent bonds
crystalline solid
solid in which the particles are arranged in a definite repeating pattern
interstitial sites
spaces between the regular particle positions in any array of atoms or ions
ionic solid
solid composed of positive and negative ions held together by strong electrostatic attractions
metallic solid
solid composed of metal atoms
molecular solid
solid composed of neutral molecules held together by intermolecular forces of attraction
vacancy
defect that occurs when a position that should contain an atom or ion is vacant
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard
Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax
College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at
http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
Adelaide Clark, Oregon Institute of Technology
6/1/2020 9.2.6 CC-BY https://chem.libretexts.org/link?119768
Crash Course Chemistry: Crash Course is a division of Complexly and videos are free to stream for educational
purposes.
Feedback
Have feedback to give about this text? Click here.
Found a typo and want extra credit? Click here.
6/1/2020 9.2.7 CC-BY https://chem.libretexts.org/link?119768
You might also like
- Types of Solids POGIL - Student VersionDocument6 pagesTypes of Solids POGIL - Student VersionJesse SchwartzNo ratings yet
- Chem M1 PDFDocument11 pagesChem M1 PDFZarylle De AsasNo ratings yet
- GENERAL CHEMISTRY 2 SolidsDocument13 pagesGENERAL CHEMISTRY 2 SolidsRicardo ErioNo ratings yet
- Lesson5 - Structure of Crystalline and Amorphous LiquidsDocument19 pagesLesson5 - Structure of Crystalline and Amorphous LiquidsLemonadeNo ratings yet
- General Chemistry 2: Learning Activity SheetDocument55 pagesGeneral Chemistry 2: Learning Activity Sheetmary joy nemenzoNo ratings yet
- Che 513 Introduction To Polymer EngineeringDocument19 pagesChe 513 Introduction To Polymer EngineeringHappyNo ratings yet
- CHEM-Types of SolidDocument4 pagesCHEM-Types of SolidMark Joseph PulintanNo ratings yet
- The Structures of Crystalline SolidsDocument76 pagesThe Structures of Crystalline SolidsgingerootNo ratings yet
- Saint Ferdinand College Sta. Ana Street, City of Ilagan, Isabela Senior High School DepartmentDocument5 pagesSaint Ferdinand College Sta. Ana Street, City of Ilagan, Isabela Senior High School DepartmentAngela AlejandroNo ratings yet
- Scientific MethodDocument26 pagesScientific Methodclarisse.ionicNo ratings yet
- Unidad 61Document36 pagesUnidad 61Eloisa OvandoNo ratings yet
- Day 3Document18 pagesDay 3孟宪南No ratings yet
- Classes of Crystalline Solids 4 Types of CrystalDocument5 pagesClasses of Crystalline Solids 4 Types of Crystaldellrubion011No ratings yet
- Activity 4 Properties of SolidDocument6 pagesActivity 4 Properties of Solidjoninna monesNo ratings yet
- Properties of Liquids and SolidsDocument33 pagesProperties of Liquids and SolidsNicolette BingtanNo ratings yet
- Physical Chemistry XiiDocument67 pagesPhysical Chemistry XiiTANISHK YADAVNo ratings yet
- Midterm Chem86 NotesDocument9 pagesMidterm Chem86 NotessujzNo ratings yet
- CH 12 Exercises: Classification of Solids (Section 12.1)Document4 pagesCH 12 Exercises: Classification of Solids (Section 12.1)Maria Donabella OngueNo ratings yet
- A2AS CHEM REVISED Support 20837Document6 pagesA2AS CHEM REVISED Support 20837Tianming KingsleyNo ratings yet
- General Chemistry 2 Lesson 3 Types of SolidsDocument11 pagesGeneral Chemistry 2 Lesson 3 Types of SolidsYeji SeoNo ratings yet
- 1.3 Revision Guide Bonding AqaDocument3 pages1.3 Revision Guide Bonding AqaPragna AnanthNo ratings yet
- Chemical Bonding SummaryDocument8 pagesChemical Bonding SummaryKiara LimNo ratings yet
- CRYSTALDocument2 pagesCRYSTALjeanzennveramaembalmesNo ratings yet
- Bonding in Solids SummaryDocument2 pagesBonding in Solids SummaryarachnidkatNo ratings yet
- Network Covalent BondingDocument2 pagesNetwork Covalent BondingRico OnrubiaNo ratings yet
- Gen Chem 2 Lesson 3 Nature of SolidsDocument27 pagesGen Chem 2 Lesson 3 Nature of SolidsJolo Allexice R. PinedaNo ratings yet
- Lesson 10.2 The Solid StateDocument14 pagesLesson 10.2 The Solid StatefitriNo ratings yet
- Chapter 15Document1 pageChapter 15api-373649599No ratings yet
- New Note Chapter 9 Structures and Properties of Substances - 2020 - Student VersionDocument46 pagesNew Note Chapter 9 Structures and Properties of Substances - 2020 - Student VersionkarinhyhoNo ratings yet
- 1 SolidsDocument23 pages1 SolidsSparshika KhannaNo ratings yet
- Xture of SolidsDocument12 pagesXture of Solidsmuonekechibukeleonard52No ratings yet
- The Solid State PDFDocument69 pagesThe Solid State PDFVNS TechNo ratings yet
- The Solid State ModifiedDocument70 pagesThe Solid State ModifiedR220603 THAMMINENI MALLIKARJUNANo ratings yet
- Classification of Crystaline Solids-Sneha LathaDocument18 pagesClassification of Crystaline Solids-Sneha LathaTarun YadavNo ratings yet
- C2: Structure, Bonding and The Properties of Matter: Key ConceptsDocument9 pagesC2: Structure, Bonding and The Properties of Matter: Key ConceptsMrs S Baker100% (1)
- Giant CovalentDocument25 pagesGiant CovalentPaarth BansalNo ratings yet
- L04 Secondary Bonding and Intro To Crystal StructuresDocument16 pagesL04 Secondary Bonding and Intro To Crystal StructuresVivek vermaNo ratings yet
- L04 Secondary Bonding and Intro To Crystal StructuresDocument16 pagesL04 Secondary Bonding and Intro To Crystal StructuresVivek vermaNo ratings yet
- L04 Secondary Bonding and Intro To Crystal StructuresDocument16 pagesL04 Secondary Bonding and Intro To Crystal StructuresVivek vermaNo ratings yet
- CH4 Atoms CombiningDocument4 pagesCH4 Atoms CombiningHazim AlJabrNo ratings yet
- 04 Crystalline SolidsDocument17 pages04 Crystalline SolidsRyan M. RamlawiNo ratings yet
- Ionic Bonding - de SilvaDocument7 pagesIonic Bonding - de SilvaDeniel Siapo de SilvaNo ratings yet
- When Atoms Meet: Chemical BondingDocument88 pagesWhen Atoms Meet: Chemical BondingWilsonNo ratings yet
- Bonding and Properties of Solids Worksheet Solutions 1kadax6Document4 pagesBonding and Properties of Solids Worksheet Solutions 1kadax6Mel Patricia M. CabreraNo ratings yet
- Giant Covalent BondDocument38 pagesGiant Covalent BondArifNo ratings yet
- Chemical StructureDocument6 pagesChemical StructuremonkeysaltaccNo ratings yet
- The Nature of SolidsDocument11 pagesThe Nature of SolidsnsuperticiosoNo ratings yet
- Solid State - PLPN MhtCetDocument42 pagesSolid State - PLPN MhtCetsiddheshmundlik6No ratings yet
- Introduction To Solid State: Dr. Amiya PriyamDocument82 pagesIntroduction To Solid State: Dr. Amiya PriyamVishal VaibhavNo ratings yet
- Class 12 Chemistry Revision Notes The Solid StateDocument21 pagesClass 12 Chemistry Revision Notes The Solid StateAfreen AnzNo ratings yet
- Module-Ii Chemical Bonding: General Chemistry CHEM-1001Document193 pagesModule-Ii Chemical Bonding: General Chemistry CHEM-1001Shivansh SharmaNo ratings yet
- The Metallic BondDocument6 pagesThe Metallic BondndoloivankaNo ratings yet
- W3W4 BTD1123 Chapter 2 Mat Structure N BondingDocument62 pagesW3W4 BTD1123 Chapter 2 Mat Structure N BondingHakim ShahmiNo ratings yet
- Structure and Properties of WaterDocument6 pagesStructure and Properties of WaterBrendan Lewis DelgadoNo ratings yet
- 6.1 SolidDocument30 pages6.1 SolidAnisha Syazwana Binti RoslyNo ratings yet
- Describe The Differences in Volatility, Solubility and Electrical Conductivity Between Ionic and Covalent CompoundsDocument6 pagesDescribe The Differences in Volatility, Solubility and Electrical Conductivity Between Ionic and Covalent Compoundsmadhuri pawarNo ratings yet
- Revision Chem Bonding NotesDocument9 pagesRevision Chem Bonding Notesrania samirNo ratings yet
- Assignment - 2Document15 pagesAssignment - 2padhiyararpitaNo ratings yet
- Appendix 04: Critical Values For T-Test: ContributorsDocument1 pageAppendix 04: Critical Values For T-Test: ContributorsGraviton Manzano OlarteNo ratings yet
- Appendix 03: Single-Sided Normal Distribution: GreenDocument3 pagesAppendix 03: Single-Sided Normal Distribution: GreenGraviton Manzano OlarteNo ratings yet
- Appendix 01: Normality: ContributorsDocument1 pageAppendix 01: Normality: ContributorsGraviton Manzano OlarteNo ratings yet
- Periodic Table of The ElementsDocument1 pagePeriodic Table of The ElementsGraviton Manzano OlarteNo ratings yet
- Worksheet CHM 130 Conversion Practice ProblemsDocument2 pagesWorksheet CHM 130 Conversion Practice ProblemsGraviton Manzano OlarteNo ratings yet
- The Barbarism of Specialisation by Jose Ortega y GassetDocument6 pagesThe Barbarism of Specialisation by Jose Ortega y GassetGraviton Manzano OlarteNo ratings yet
- Edexcel A2 Chemistry Paper 6Document148 pagesEdexcel A2 Chemistry Paper 6AbdulRahman MustafaNo ratings yet
- Reff (IATA 2013 Section 3 3.0.2)Document5 pagesReff (IATA 2013 Section 3 3.0.2)Okshella reno FajrianiNo ratings yet
- prEN 14700-FD-2013-11-Consumiveis para Revestimentos DurosDocument15 pagesprEN 14700-FD-2013-11-Consumiveis para Revestimentos DurosRicardo FernandesNo ratings yet
- (Skt3013) Laboratory Report - Exp 4Document10 pages(Skt3013) Laboratory Report - Exp 4Fiona TiwonNo ratings yet
- Lecture 5 Carboxylic Acids and Esters-2Document113 pagesLecture 5 Carboxylic Acids and Esters-2JowayriyyahNo ratings yet
- Halox SZP-391: Technical DataDocument1 pageHalox SZP-391: Technical DataArturo BarjauNo ratings yet
- Redox Titration With Potassium PermanganateDocument1 pageRedox Titration With Potassium PermanganatekayraozlemNo ratings yet
- Assessment of Chemical Conversion Coatings For The Protection of Aluminium Alloys A Comparison of Alodine 1200 With Chromium-Free Conversion Coatings PDFDocument61 pagesAssessment of Chemical Conversion Coatings For The Protection of Aluminium Alloys A Comparison of Alodine 1200 With Chromium-Free Conversion Coatings PDFCemalOlgunÇağlayanNo ratings yet
- SpectrometerDocument17 pagesSpectrometerMazzel Lou BaelNo ratings yet
- 6.0 Gavimetric AnalysisDocument1 page6.0 Gavimetric AnalysisSister RislyNo ratings yet
- LectroPol-5 BrochureDocument4 pagesLectroPol-5 BrochureRonak ShahNo ratings yet
- Introduction For Experiment 6, Preparation of Bis (Acetylacetonato) Copper (II)Document1 pageIntroduction For Experiment 6, Preparation of Bis (Acetylacetonato) Copper (II)刘象No ratings yet
- ATEST ALU LIM 2mmDocument1 pageATEST ALU LIM 2mmNedžad MehmedbegovićNo ratings yet
- University of Central Punjab Multan Campus: Assignment #02 Roll # 58 Subject: Luminescence Spectroscopy andDocument4 pagesUniversity of Central Punjab Multan Campus: Assignment #02 Roll # 58 Subject: Luminescence Spectroscopy andAdnan AliNo ratings yet
- Engineeing Solutions: Rev 01 - Nov 2014Document22 pagesEngineeing Solutions: Rev 01 - Nov 2014aliNo ratings yet
- Chemical ApplicationsDocument4 pagesChemical ApplicationsBinit DuttNo ratings yet
- Class 8 - Science - CH - 3 - Metals - Non Metals - NotesDocument4 pagesClass 8 - Science - CH - 3 - Metals - Non Metals - Notes8eanjanimaitreyNo ratings yet
- Is 3752 2005Document19 pagesIs 3752 2005Ganciarov MihaelaNo ratings yet
- Innospec Iselux LQ CLR SBDocument6 pagesInnospec Iselux LQ CLR SBYap Ming ZheNo ratings yet
- Class 8 Cbse Chemistry Sample Paper Term 1 Model 2Document2 pagesClass 8 Cbse Chemistry Sample Paper Term 1 Model 2Sunaina RawatNo ratings yet
- From Pepper (Piperonal) To MdaDocument14 pagesFrom Pepper (Piperonal) To MdaM. Shehryar KhanNo ratings yet
- Mcqs On LipidsDocument4 pagesMcqs On LipidsFEROZ KHANNo ratings yet
- Topic 2 Exercise 6 - More Complex CalculationsDocument1 pageTopic 2 Exercise 6 - More Complex Calculationsupeka weerasinghaNo ratings yet
- Acidity-Alkalinity of Halogenated Organic Solvents and Their AdmixturesDocument3 pagesAcidity-Alkalinity of Halogenated Organic Solvents and Their AdmixturesShaker QaidiNo ratings yet
- Paint SpecificationDocument2 pagesPaint SpecificationsanjuranjNo ratings yet
- Fouling Corrosion in Aluminum Heat ExchangersDocument7 pagesFouling Corrosion in Aluminum Heat Exchangersهدوء السماءNo ratings yet
- Topic 3 Acids Bases Salts SolutionDocument13 pagesTopic 3 Acids Bases Salts Solutionindira.seebachanNo ratings yet
- Senior One ChemistryDocument10 pagesSenior One ChemistryMwesigwa JoshuaNo ratings yet
- Sist Iso 1871 2011Document9 pagesSist Iso 1871 2011pratita ameliaNo ratings yet
- SCPF-PETW-LIS-P-01001 - Rev0 - Utility Consumption List - CPFDocument3 pagesSCPF-PETW-LIS-P-01001 - Rev0 - Utility Consumption List - CPFSEGUNNo ratings yet