Professional Documents
Culture Documents
Behavior and Maintenance of Captive White-Winged Vampire Bats, Diaemus Youngi
Behavior and Maintenance of Captive White-Winged Vampire Bats, Diaemus Youngi
Uploaded by
Islam Ausraf RajibOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Behavior and Maintenance of Captive White-Winged Vampire Bats, Diaemus Youngi
Behavior and Maintenance of Captive White-Winged Vampire Bats, Diaemus Youngi
Uploaded by
Islam Ausraf RajibCopyright:
Available Formats
BEHAVIOR AND MAINTENANCE OF CAPTIVE WHITE-WINGED
VAMPIRE BATS, DIAEMUS YOUNGI
W. A. SCHurr, JR., F. MURADALI, N. MONDOL, K. JOSEPH, AND K. BROCKMANN
Natural Science Division, Southampton College, Long Island University,
Southampton, NY 11968 (WAS)
17 Winsor Road, Valsayn Park, Trinidad (FM)
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
National Animal Disease Center, Caroni, North Bank Road, Trinidad (NM, KJ)
Department of Ecology and Systematics, Corson Hall, Cornell University, Ithaca, NY 07003 (KB)
Procedures for transportation and maintenance of the white-winged vampire bat, Diaemus
youngi, are documented for the first time. Contrary to previous reports, D. youngi has been
maintained on a diet of defibrinated bovine blood supplemented weekly with fresh chicken
blood. Gregarious by nature, D. youngi exhibits dominance-hierarchy behavior, and behav-
ioral patterns unreported in other species of bats. Although primarily arboreal with regard
to feeding behavior, D. youngi demonstrates the ability to feed terrestrially-a behavior
documented for the first time. Observations of our captive colony contradict anecdotal and
previously published information on feeding and behavior. Differences in feeding behavior
between D. youngi and the common vampire bat, Desmodus rotundus, appear to be related
to selection of prey (arboreal versus terrestrial prey, respectively). In places where these
vampire bats coexist, resource partitioning may serve to reduce competition.
Key words: Diaemus, Desmodus, vampire bats, captive maintenance, behavior
Reports on the maintenance and behavior unstudied. Similarly, relatively little is
of captive vampire bats (Phyllostomidae, known about the biology of the white-
Desmodontinae) generally are restricted to winged vampire bat, Diaemus youngi
the common vampire bat, Desmodus rotun- (Greenhall and Schutt, 1996). As with D.
dus (Dickson and Green, 1970; Ditmars and ecaudata, there is consensus that D. youngi
Greenhall, 1935; Greenhall, 1965; Trapido, prefers avian blood (Gardner, 1977; Good-
1946; Wimsatt, 1962a, 1962b; Wimsatt and win and Greenhall, 1961; Sazima and Uie-
Guerriere, 1961). D. rotundus is common da, 1980; Uieda et al., 1992).
in the wild (Ditmars and Greenhall, 1935; There are few reports on the maintenance
Greenhall, 1965), easily maintained, and of D. youngi in captivity, and bats in these
long-lived in captivity (Dickson and Green, studies were not transported out of the
1970; Trapido, 1946; Wimsatt, 1962a, country of origin. Two adult females, cap-
1962b; Wimsatt and Guerriere, 1961). tured and maintained in Brazil, were al-
Wimsatt (1978) maintained a colony of D. lowed to feed on a diet of live chickens
rotundus at Cornell University for >20 (Uieda and de Araujo, 1987), and four
years and reported that at least one individ- adults fed on chickens, Gallus gallus, do-
ual survived> 19 years. The hairy-legged mestic pigeons, Columba livia, and collared
vampire bat, Diphylla ecaudata, is less doves, Streptopelia decaocto (Uieda et al.,
abundant compared with D. rotundus and 1992). Lack of observational data on D.
reportedly difficult to maintain in captivity youngi probably can be attributed to two
(Greenhall, 1976; Villa-R., 1967) although major factors: the relative rarity of D. youn-
Hoyt and Altenbach (1981) maintained gi in the wild and reports of difficulty main-
them successfully on a diet of blood from taining D. youngi in captivity. Goodwin and
live chickens. D. ecaudata remains largely Greenhall (1961) reported that D. youngi
Journal of Mammalogy, 80(1):71-81, 1999 71
72 JOURNAL OF MAMMALOGY Vol. 80, No.1
would not accept bovine blood in captivity, Presence or absence of a cartilagenous zone in
whereas Pye (1967) reported that D. youngi the epiphyseal region of the fourth metacarpal-
took small quantities of bovine blood but phalangeal joint was used for determination of
did not survive without avian blood. Con- relative age (Kunz and Anthony, 1982). For pur-
poses of identification and record keeping, fore-
trary to Goodwin and Greenhall (1961), we
arms of bats were fitted with individually num-
found that captive D. youngi readily accepts
bered metal bands soon after capture.
bovine blood. Bovine blood facilitates Before and during transport, we complied
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
maintenance of captive colonies, as it is rel- with all rules and regulations concerning collec-
atively easy to obtain in large quantities, tion, export, and import of live animals. Regu-
can be stored frozen for extended periods lations differ from country to country and spe-
of time, and eliminates the requirement for cial care should be taken to insure that all re-
a daily supply of live blood donors. It has quired permits are completed and available for
become a dietary staple of captive colonies presentation during transit. Before our trip, we
of D. rotundus (Dickson and Green, 1970; contacted the United States Fish and Wildlife
Greenhall, 1965; Wimsatt and Guerriere, Service's Office of Management Authority
1961). (4401 North Fairfax Drive, Room 432, Arling-
ton, VA 22203), and we were instructed that
We report here observations on the be-
upon entering the United States with the bats,
havior and maintenance in captivity of 12
we were to declare them and present United
D. youngi. Many behavioral observations States Customs Form 3177. Specimens of D.
are reported here for the first time, and this youngi and D. rotundus, including several newly
is the first detailed account of a successful captured individuals, were transported via com-
maintenance program for captive D. youngi. mercial airline from Port of Spain, Trinidad, to
It should be noted that the daily mainte- New York City, and then by car to Ithaca, New
nance program we describe was developed York. A wooden travel crate was constructed (90
over the past 25 years by workers at the by 90 by 90 cm), which securely held a pair of
National Animal Disease Center, Caroni, wire-mesh, stainless steel, cages. Airholes (0.5
Trinidad. Although this work primarily is cm diameter) were drilled into the sides of the
crate, which were conspicuously labeled (e.g.,
concerned with D. youngi, much of the
Live Animals, Do Not Open, This End Up). On
maintenance program described here (e.g.,
several evenings before the trip, bats were
transportation and housing) can be applied placed into the wire cages and the cages fitted
to D. rotundus. We speculate that captive into the crate to acclimate the animals to the
colonies of D. ecaudata require a diet com- transport container. The bottom of the cage con-
posed solely of avian blood. Several refer- tained an aluminum tray onto which absorbant
ences provided valuable information on the material (newspaper) was placed. Before sealing
care of captive D. rotundus (Dickson and the crate for transport, bats were fed and placed
Green, 1970; Greenhall, 1976; Greenhall together into one of the wire cages (11 D. ro-
and Schmidt, 1988; Wimsatt and Guerriere, tundus were transported in the second wire
1961). cage), and cages were fitted into the crate. The
crate lid was sealed with wood screws. All 23
MATERIALS AND METHODS of the bats survived transportation from their
country of origin; the crate was sealed for 18 h.
Twelve adult D. youngi (10 males, 2 females) Captive D. rotundus and D. youngi were
were collected 17 Februrary-26 July 1993 by maintained at Cornell University from July 1993
mist netting from the following locations in to September 1996. In September 1996, bats
Trinidad (Guayaguayare, Mayaro; Ortoire, May- were transported to the Burnett Park Zoo, Syr-
aro; Matura, St. Andrew; Raghunan Road, Ca- acuse, New York. At Cornell University, access
roni, Tabaquite, Caroni). All bats were main- to the bat colony was restricted to research and
tained at the National Animal Disease Center, animal-care personnel. Specimens, separated by
Caroni, Trinidad, before transport to Cornell genus, were housed in two, stainless-steel,
University, Ithaca, New York, on 27 July 1993. small-animal cages (56 by 51 by 43 cm). News-
1999 SCHurr ET AL.-DlAEMUS YOUNG1 IN CAPTIVITY 73
paper was used as an absorbent on the cage floor fibrinated bovine blood. That blood was ob-
and changed daily. A warm damp sponge was tained monthly at a local slaughterhouse from
used to remove blood and excreta from cage freshly killed domestic cattle. A clean, plastic,
walls, and cages were scrubbed with cage clean- 7.S-1 container was used to collect blood. To pre-
ing detergent on a weekly basis. vent clots during storage or feeding, blood was
All research and animal-care personnel that fibrinated immediately by manual agitation. A
had any contact with our vampire bats had first metal kitchen colander was used to skim off
undergone a pre-exposure prophylaxis for rabies clots, and the newly defibrinated blood was
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
virus. Although our D. youngi became tame and stored for transport in 3.78-1 plastic jars. In the
did not attempt to bite us after their 1st week of laboratory, the blood was transferred to individ-
captivity, we continued to use thick, loose-fit- ual 473-ml glass bottles. We stored enough in
ting, leather gloves when handling them. each bottle to feed each bat 30 mi. That volume
Room temperature in our laboratory was 21- was more than sufficient because it was about
24°C; relative humidity was variable at 20-S0%. twice the amount normally consumed by our
Bats were maintained on a cycle of 12 h of in- bats. Bottles of blood were frozen at -20°C until
direct flourescent ceiling light (0600-1800 h) use-keeping the bottle's lid loose to prevent
and 12 h of darkness. This schedule approxi- glass bottles from cracking when frozen. On the
mated the day-night light conditions in Trinidad. day that the blood was used, a bottle was thawed
We had few problems with illness in our col- slowly, and blood was poured into a plastic ice-
onies. Occasionally, however, bats presented cube tray and served at room temperature. We
some or all of the following symptoms: lethargy, filled one ice-cube tray depression (ca. 30 ml)
dehydration, bloated abdomen, sneezing, and with blood for each bat. Because D. youngi
vomiting of a previous blood meal. We treated feeds arboreally in the wild (Gardner, 1977;
our bats in the following manner. At the first
Goodwin and Greenhall, 1961; Sazima and Uie-
sign of illness, the bat was isolated in a wire-
da, 1980; Uieda et aI., 1992), we elevated the
mesh travel cage draped in a clean towel to re-
feeding tray ca. 30 cm off the cage floor with a
duce drafts. Ambient temperature was increased
wooden block. Bats were fed in the late after-
(ca. SoC) with an electric space heater. If dehy-
noon or evening, and the tray was removed and
dration was observed, the bat was rehydrated
cleaned the next morning.
daily with a 1.0-ml subcutaneous injection of
In our study of feeding capacity, the average
0.9% saline solution. Animals were treated as
daily intake of blood was determined, over a 30-
dehydrated if the loose skin covering the region
day period, for isolated D. youngi. Eight indi-
between their shoulder blades did not spring
viduals were used (four times each) in 32 trials.
back into place immediately after being gently
pinched by the handler. An antibiotic (10 mg Thirty milliliters of blood was offered in a spill-
Baytril/kg-Bayer Corporation, Shawnee Mis- proof feeding container. The next day, the
sion, KS) was administered subcutaneously, amount of blood remaining was subtracted from
once per day. Subcutaneous injections were the starting amount to yield the amount con-
made beneath the loose skin of the upper back. sumed. A correction factor of 2 ml was applied
In the evening, the bat was allowed to feed on to account for volume loss due to evaporation
a live chicken. This treatment regime proved ex- and blood that clung to the bat during feeding.
tremely successful, and all bats treated in this Although our bats were placed into a stain-
manner recovered within S days. At that time, less-steel feeding cage with the chickens, >SO
treatment was discontinued, and the bat was re- arboreal feeding sessions were carried out in a
introduced into the colony. 1.8 by 3.0-m screened enclosure, with chickens
Our feeding regime for D. youngi was iden- roosting on a branch. During preparation for
tical to one that had been developed and suc- those sessions, a chicken was placed onto the
cessfully employed to maintain bats in Trinidad upper section of a branch (inverted L-shape, ca.
(Muradali et aI., 1993). Specifically, bats were 1.3 m in length, 4 cm in diameter), the lower
fed bovine blood six times per week and offered section of which was attached to a stand con-
live chickens once per week. Contrary to pre- structed with two sections of S- by lO-cm pine
vious reports (Goodwin and Greenhall, 1961; studs. With the room lights lowered or turned
Pye, 1967), our D. youngi readily accepted de- off, a single D. youngi was placed on the lower
74 JOURNAL OF MAMMALOGY Vol. 80. No.1
part of the branch (ca. 15 cm off the floor) in a indicates that captive vampire bats may not
head-up position. be as sensitive to relative humidity as other
One evening per week, two specimens of D. species of bats kept in captivity (e.g., ves-
youngi were placed in a stainless-steel feeding pertilionids). Racey (1972) reported that
cage (55 by 50 by 42.5 cm). A single subadult low humidities can be injurious to wing
chicken (9-10 weeks old) was placed in the
membranes. Although wing injuries have
cage. Bats were permitted to feed on the chicken
not been noted in our laboratory, humidity
overnight.
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
For observations of terrestrial feeding behav- should be given careful consideration in
ior a large wire-mesh cage (51 by 84 by 37 cm) studies where bats are required to fly exten-
was used. Twenty terrestrial feeding sessions sively.
were observed, during which a single bat was Ectoparasite infestation was observed in
placed into the cage with one adult or subadult newly captured vampire bats, but this prob-
chicken. Because there were no perches in the lem disappeared within a month. As re-
cage, the chickens stood on the cage floor. Room ported in previous studies (Dickson and
lights were turned off and a small flashlight fit- Green, 1970; Wimsatt and Guerriere, 1961),
ted with a red-gel filter was used to observe ter- this problem disappeared gradually over the
restrial feeding behavior. Sex and age of chick- course of several weeks, probably due to
ens and the identification number of the bat used
the disruption of ectoparasite lifecycles in
were noted. Behavior during those sessions was
captive conditions (Wimsatt and Guerriere,
recorded in a notebook.
1961). Information on ectoparasites and en-
RESULTS AND DISCUSSION doparasites of D. rotundus exists (Mendez,
1988), but data are fragmentary with regard
Wimsatt et al. (1973) designed a cage for to D. youngi (Mendez, 1988).
vampire bats with a novel and time-saving Cultures of the bovine blood were tested
sanitation system, but satisfactory housing (Cornell University, Department of Pathol-
can be provided with less elaboration. Basic ogy) and indicated presence of bacteria that
requirements for cages are that they prevent may have caused occasional gastrointestinal
bats from escaping, allow bats to move distress observed in several of our bats.
about and roost comfortably, and be easy to Tests to determine presence of Salmonella
clean. Because excreta is tar-like and cor- were negative.
rosive, housing constructed of stainless Data on the demeanor of vampire bats in
steel or plastic is advisable. captivity is variable. Some authors have
In a previous study (Uieda and de Ar- found, as we have, that D. rotundus does
aujo, 1987), D. youngi was maintained at not tame in captivity (Dickson and Green,
temperatures similar to those in our labo- 1970), while others report that D. rotundus
ratory. In Trinidad, our bats were caged in tames readily (J. S. Altenbach, pers. comm.;
a open-air facility where temperatures gen- Novick, 1963; Wimsatt and Guerriere,
erally were 21-29°C. Greenhall (1965) 1961). D. youngi has been described as ag-
maintained D. rotundus and D. youngi in gressive in captivity (Uieda and de Araujo,
Trinidad at similar temperatures. Although 1987), while we have found the opposite to
it has been reported that vampire bats re- be true. Like D. rotundus, D. youngi ap-
quire high relative humidity (60% mini- pears to be gregarious by nature and can be
mum) in captivity (Joermann, 1988), D. housed in a group dwelling. There have
youngi does not appear sensitive to low hu- been no problems maintaining mixed-sex
midity, and we have maintained D. youngi populations, although D. youngi does ap-
and D. rotundus at relative humidities of pear to exhibit dominance hierarchies. The
20-50%. This agrees with previous data on following behaviors were noted in Trinidad,
maintenance of captive specimens of D. ro- when a new animal was introduced into an
tundus (Wimsatt and Guerriere, 1961) and established group and at Cornell University,
1999 SCHUTT ET AL.-D1AEMUS YOUNG1 IN CAPTIVITY 75
the day after their arrival. In both instances, emission of a fine spray of liquid, which has
a male that had been captured recently was a strong musky odor. We have observed
approached by another male. These bats are musk spraying only during threat response.
hereafter referred to as the newcomer and We suggest that this aerosol may be used to
the dominant male, respectively. The bats discourage predators and may serve a role
faced each other and both displayed a rep- in territory marking and individual recog-
ertoire of threat behavior. After rising up nition. Enlarged oral glands and musk
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
into an elevated stance, a distinctive hissing spraying have not been reported in D. ro-
was accompanied by other audible vocali- tundus or D. ecaudata. There have been
zations varying in frequency and intensity. studies on anatomy and histochemistry of
Forelimbs, held in a closed-wing position, the salivary glands of D. rotundus (Di-
were used for parrying. That behavior, Santo, 1960) and the salivary antihemostat-
which was accompanied by feints, lunges, ic factors of D. rotundus and D. youngi
and head bobbing, lasted ca. 20 s. In the (Hawkey, 1966, 1967). However, there
Trinidad episode, the newcomer then have been no studies on the anatomy of sal-
crouched quietly, partially opened his ivary glands in D. youngi or D. ecaudata.
mouth and exposing a pair of enlarged oral Our vampire bats spent considerable time
glands. The dominant male then inserted his roosting. During that time, they clung to
tongue into the mouth of the newcomer for each other with claws and thumbs of their
several seconds. At Cornell University, af- hind limbs. Hind limbs not in contact with
ter a similar face-off (in this instance there conspecifics generally were used to grasp a
was no tongue insertion), the dominant branch or the cage ceiling. Number of bats
male approached the newcomer from the roosting together varied but most often,
rear. With both bats vocalizing loudly, the they clung together in one dense mass. Dur-
dominant male partially extended a wing, ing roosting, we frequently observed an in-
wrapped it around the back of the newcom- dividual apparently biting the bat hanging
er, and climbed over it. This took several adjacent and ventral to it. Upon examina-
seconds, after which time the bats ceased tion, however, we learned that those were
their vocalizations and moved away from not actually bites but rather one bat took a
each other. That stylized fighting behavior loose fold of skin from a cage mate (usually
was observed on a number of occasions in from the upper back or dorsal surface of the
our lab especially during sessions with pairs neck, the nape) into its mouth (Fig 1a).
of bats feeding on live chickens. Unlike the There, the skin fold was held securely for
violent and bloody clashes reported in stud- several seconds. Bats on the receiving end
ies on D. rotundus during roost-defense en- of that mouth grasp did not appear dis-
counters (Wilkinson, 1985, 1988), those be- turbed. Upon examination of those animals,
tween D. youngi have not resulted in blood- neither wounds nor hair loss were observed.
shed. This behavior has not been reported in other
Diaemus youngi possesses two large oral studies on D. youngi in the wild (Sazima
glands whose function is unknown (Good- and Uieda, 1980; Uieda et aI., 1992) or in
win and Greenhall, 1961; Greenhall, 1988). captivity (Uieda and de Araujo, 1987; Uie-
When disturbed (such as we have seen dur- da et al., 1992).
ing dominance-hierarchy behavior or at Mouth grasping is most reminiscent of
times during handling), D. youngi opens its behavior seen in non-fiying neonatal bats as
mouth, and these large glands can be clear- they cling onto their mother's body with
ly observed filling the caudolabial part of their mouths, thumbs, and hindlimbs. We
the oral cavity. A peculiar hissing vocaliza- suggest that mouth grasping is a component
tion, described by Goodwin and Greenhall of dominance-hierarchy behavior. A. Stem
(1961) as a "pssst," is accompanied by (pers. comm.) reported similar behavior in
76 JOURNAL OF MAMMALOGY Vol. 80, No.1
al., 1992), live birds were used as the sole
source of blood. Although our bats readily
accepted defibrinated bovine blood, we be-
lieve that citrated blood (Wimsatt and Guer-
riere, 1961), also would be acceptable. Be-
cause these bats feed primarily on avain
blood in the wild, we chose to provide them
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
with a weekly supplement of blood from a
live chicken. While feeding from an ele-
vated ice-cube tray, bats hung from the cage
ceiling by their hind limbs and extended
their upper bodies toward the tray (until
their mouths were just above the level of
the blood). As the bat fed, the tongue was
extended into the blood and withdrawn at a
rate of ca. 4 licks/so Feeding usually lasted
ca. 15 min and fighting was never observed
during those sessions.
Observations on feeding capacity of D.
rotundus in captivity (Wimsatt and Guerri-
ere, 1961) indicated that average daily in-
take of blood by two groups was 15.3 and
15.6 mI. In our study, mean daily intake for
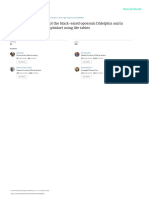
FIG. I.-a) Mouth-grasping behavior by Diae- D. youngi was 16.4 ± 3.6 (SD) mlIday, but
mus youngi. While roosting or during domi- there were differences in the method of
nance-hierarchy behavior, individuals often use
those two studies (e.g., the study of D. ro-
their mouths to grasp cage mates by the loose
skin between the shoulders or on the nape of the
tundus was carried out over a full year but
neck. b) D. youngi mounting a young female animals were only fed 6 days/week). Wil-
chicken dorsally prior to feeding. Rather than kinson (1984) noted that D. rotundus did
becoming agitated, female chickens mounted in not necessarily feed every night and some
this fashion assume a crouched posture similar bats received blood regurgitated by other
to that observed during copulatory behavior; we individuals. Blood sharing has not been re-
suggest that the position and body weight of the ported in D. youngi.
bat trigger submissive behavior in female chick- Sazima and Uieda (1980) reported on the
ens. feeding behavior of D. youngi on free-rang-
ing poultry (chickens, Guinea fowl, and tur-
keys), but were unable to observe the actual
Pteropus vampyrus during dominance biting behavior and the prey's reaction. Uie-
fights and copulations in several species of da and de Araujo (1987) documented some
Pteropus. Additionally, mouth grasping ap- aspects of maintenance of D. youngi and D.
pears to assist D. youngi in stabilizing its ecaudata in captivity but did not go into
body during roosting, especially during great detail concerning feeding behavior.
shifts in position of hind limbs. Mouth- Uieda et al. (1992) studied arboreal feeding
grasping behavior was not observed in our on chickens and smaller birds (pigeons and
D. rotundus nor has it been reported in oth- doves). They described two feeding pos-
er microchiropterans (K. E Koopman, pers. tures used by bats, hanging and quadrupe-
comm.). dal, and stressed the importance of diameter
In previous studies on D. youngi in cap- of branch as a limiting factor in attacks by
tivity (Uieda and de Araujo, 1987; Uieda et D. youngi on small birds.
1999 SCHUTT ET AL.-DlAEMUS YOUNG1 IN CAPTIVITY 77
The following description characterizes a lowing events occurs to complete the bite.
typical arboreal feeding session in our lab- Either a series of quick backward steps are
oratory and in Trinidad (Muradali et aI., made by the bat or the chicken shifts its
1993). Holding on with its thumbs and hind position slightly on the branch and the digit
feet, D. youngi clings quietly with its ven- is pulled away from the bat's mouth. In the
tral body surface held tightly to the branch. majority of bites, chickens show little, if
During this period, which lasts :::; 10 min, any indication that they have been disturbed
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
the bat raises its head occasionally, sniffs at by the bite.
the air with its mouth partially open, and Bites appear as small crater-shaped in-
observes the chicken perched above it. At dentations that extend into the dermis-a
this time, the bat moves to the underside of layer richly supplied by blood vessels. Bites
the branch and slowly climbs closer to the inflicted by D. rotundus appear similar and
perching chicken. D. youngi advances one have been well characterized (Greenhall,
limb at a time (with the grip on the branch 1988). In D. rotundus, canine teeth are used
maintained by the three non-moving limbs) to shave the fur around a wound site. The
always keeping the branch between itself lobed lower incisors secure a firm hold on
and the underside of the chicken. After po- the skin while the concave upper incisors
sitioned beneath the chicken's digits (ca. 1 remove a divot of flesh. D. youngi may use
min), the bat situates itself so that its head its canines in a similar manner to shave
is close to a single digit of the roosting bird. feathers, but this has not been reported and
On most occasions, the bat positioned its we did not observe this behavior.
head next to the posteriorly directed hallux After the bite is inflicted, D. youngi re-
(digit 1). It appears that feeding from this turns to the wound and resumes the licking
digit keeps the bat concealed under its prey behavior at a rate of ca. 4 licks/so Within
to a greater degree than would occur if an 30 s of the bite, blood flows freely from the
anteriorly directed digit (digits 2-4) was wound. Blood is conducted into the bat's
fed upon. Moving its mouth to the digit, the mouth via two grooves on the ventral sur-
bat's tongue is extended and withdrawn (in face of the tongue (Uieda, 1986) through a
'a piston-like manner), and the potential bite gap between the two pairs of lower incisors,
area is licked at a rate of ca. 2 licks/so and above a V-shaped groove in the lower
It is not known if saliva of vampire bats lip. Most feeding sessions last ca. 15 min
contains anesthetic or enzymatic compo- and may stop temporarily if the bird
nents to aid in preparation of bites or if lick- changes positions or becomes alarmed.
ing softens the keratinized reticulate scales When this occurs, the bat may back off,
that cover the surface of the digit. The bite- concealing itself under the branch, until the
preparation period lasts from 10 s to 2 min. bird calms. On other occasions, the bat re-
Bites were never inflicted without this prep- mains in position and appears to hide under
aration period. In all of our observations, the body of the bird. After the bird settles
immediately prior to the bite, D. youngi down, the bat re-establishes its position and
turns its head so that the midline of the head begins feeding. At no time (during either
is perpendicular to the long axis of the digit arboreal or terrestrial feeding behavior) did
being bitten. The actual bite is never initi- we observe a feeding bat make a second
ated quickly or violently but occurs when bite on the same chicken. Interruptions in
licking stops and the bat applies its partially feeding always were followed by a resump-
opened mouth to the site. At this time, it tion of feeding from the same wound. We
appears that the jaws close, driving the ra- have regularly observed periods of urina-
zor-sharp upper incisors through the epi- tion, lasting several seconds, commencing
dermis and into the dermis. This position is at ca. 5 min into the feeding session. While
maintained for 1-2 s until one of the fol- urinating, one hind limb usually is extended
78 JOURNAL OF MAMMALOGY Vol. 80, No.1
laterally and slightly downward, preventing located on digits or the posterior side of the
the bat from soiling itself. intertarsal joint. Some bites, however, were
On several occasions, bites made on the made on the comb, neck, or abdomen, and
terminal digital pad of digit 1 (ventral to the a single bite was found on the proximal pre-
proximal portion the claw) were not licked patagium of the wing.
during feeding. Instead, the protruding dig- Descriptions of terrestrial feeding behav-
ital pad was taken into the bat's mouth as ior by D. youngi are lacking. Uieda et al.
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
it fed. On two occasions, we attempted to (1992) mentioned a single observation of
dislodge the bat from the bird's digit and quadrupedal posture employed by D. youn-
found that it took a considerable amount of gi during feeding on a domestic pigeon, but
pulling for the bat to release its hold. Our it is unclear if that occurred during arboreal
attempts to dislodge the bat did not disturb or terrestrial feeding. The following is a de-
the chicken, and no further wounds were scription of a typical terrestrial feeding ses-
apparent on them after the bat was pulled sion in our laboratory. After the bat is
free. We suggest that suction (rather then placed into the cage, it uses a hopping gait
the use of teeth) was used during this pe- to position itself under the posterior part of
culiar feeding behavior. This observation the bird's body behind the feet. If the bird
lends support to the hypothesis that vampire moves about the cage, the bat hops after it,
bats may use their mouths to form a seal trying to maintain its position under and be-
around the wound when feeding (Greenhall, hind the bird. After the bird is stationary,
1988). Suction and wound licking appear to the bat approaches the posterior side of the
be two methods for obtaining blood from a metatarsus. Raising itself into an elevated
wound. Because suction was observed only quadrupedal stance, similar to that de-
when D. youngi fed on the distal digital scribed for D. rotundus (Altenbach, 1979,
pad, it appears that these feeding methods 1988; Sazima, 1978), a bite-preparation
may be dependent on location of wounds. phase is initiated. In most instances, bites
After ca. 15 min, D. youngi concluded its are made on the posterior or lateral surface
feeding session by releasing its thumbs just distal to the intertarsal joint. Larger
from their hold on the branch. After hang- scales (scutes or scutella) are partially or
ing briefly by its hind limbs, the bat re- fully removed during bites to the metatar-
leased its grip on the branch. In many in- sus. Bite infliction is similar in terrestrial
stances, flight was initiated by this method, and arboreal feeding in that the bite is com-
but on a few occasions D. youngi dropped pleted if the chicken moves away from the
directly to the ground before scrambling off stationary bat or the bat steps backward
to hide. away from the bird's leg. After blood be-
Because of the design of cages, cages gins to flow from the wouQd, the bat ap-
used during weekly supplements of diet proaches the wound and begins to feed. If
with chicken blood were not used to ob- the bird moves around the cage, the bat dis-
serve bat-bird interactions but solely for engages feeding and follows the bird by
feeding purposes. In all cases, subadult hopping behind and underneath it. Feeding
chickens placed into the feeding cage were resumes when the bird assumes a stationary
removed and euthanized after 4 h. In a pre- pose.
vious study (Uieda and de Araujo, 1987), During a number of terrestrial feeding
bats and chickens were kept together con- sessions, we noted agitated and aggressive
tinuously. Those chickens exhibited de- behavior by male chickens toward a bat.
creased activity and weight loss, and a That behavior consisted of rapid pacing
number of them died within 7-10 days. We back and forth in the feeding cage by the
examined locations of bites on carcasses of bird accompanied by pecks directed toward
chickens and observed that most bites were the bat. Inevitably, that behavior resulted in
1999 SCHUTT ET AL.-D1AEMUS YOUNG1 IN CAPTIVITY 79
the bat retreating to the cage ceiling where According to Koopman (1988), there is
it remained until removed. D. youngi were disagreement with regard to the recognition
observed feeding on male chickens on of Desmodus and Diaemus as seperate gen-
many occasions, but they seem to prefer fe- era. Handley (1976) for example, included
male chickens. Diaemus within the genus Desmodus. Enu-
In ca. 10% of terrestrial feeding sessions merating 18 morphological characters,
observed, the bat did not feed from the Koopman (1988:12) was "inclined to rec-
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
chicken's leg or digit but leaped or climbed ognize Diaemus as a valid genus along with
onto the chicken's back to feed on the head Diphylla and Desmodus." In our studies,
region. In those instances, the bat posi- we observed a number of behavioral char-
tioned itself with its body facing cranially acters, related to feeding and roosting be-
with regard to the bird. That position was havior (e.g., mouth grasping), which sup-
maintained by partially spreading (abduct- port that view.
ing) forelimbs while grasping onto dorsal The locomotor morphology of D. rotun-
feathers with both thumbs and digit claws dus was studied in detail by Altenbach
of the hind limb. (1979, 1988) and Schutt et al. (1997). Al-
Male chickens that were mounted dor- tenbach (1988) described the quadrupedal
sally by D. youngi quickly became agitated, stance of this genus as elevated. This stance
often dislodging bats from their back. When apparently allows "immediate movement in
young female chickens were mounted dor- any axis parallel to the surface or move-
sally, however, rather than becoming agi- ment away from it without repositioning of
tated, they assumed a crouched posture either pectoral or pelvic limbs" (Altenbach,
(Fig. 1b) similar to that observed during 1988:72). The elevated posture appears to
copulatory behavior. They remained in this be an adaptation for rapid terrestrial loco-
position even when moving around the motion in D. rotundus, allowing it, for ex-
cage. We suggest that the forces applied to ample, to initiate flight by jumping verti-
the female bird's back by the bat triggers cally into the air. Jumping functions to get
this innate submissive response behavior. the heavily loaded bat airborne. It also aids
Attacks on poultry roosting in trees are in predator avoidance (e.g., snakes) and
frequent in Trinidad, but there have been no may prevent the bat from being stepped on
reports of attacks on birds confined in cages by ungulate prey while feeding.
on the ground although the mesh of these In contrast, D. youngi exhibits a more
cages would allow entrance by D. youngi. crouched stance than D. rotundus. The
Our feeding experiments, however, showed body of D. youngi is held so that its center
that D. youngi are quite capable of efficient of mass is closer to the support (the ground
terrestrial feeding. Examination of stomach or branch). D. youngi has never been re-
contents of D. youngi has, in fact, shown ported to perform flight-initiating jumps in
them to feed on mammalian blood (e.g., the wild or in captivity. Additionally, we
cattle and pigs-Greenhall, 1988). Our ob- never observed this behavior during force-
servations suggest that although D. youngi platform experiments analyzing terrestrial
is capable of feeding terrestrially, it will not locomotion (Schutt et al., 1997). We sug-
do so as long as arboreal prey is available. gest that D. youngi, with its arboreal feed-
One possibility is that arboreal feeding ing habits, is under less threat from terres-
evolved to reduce competition between D. trial predators than D. rotundus. Unlike the
youngi and the terrestrially feeding D. ro- terrestrially feeding D. rotundus, D. youngi
tundus where their ranges overlap, although is in no danger of being crushed by large-
Schutt and Altenbach (1997) suggest that ungulate prey. During terrestrial feeding
arboreal feeding is a primitive behavior in sessions with D. youngi, chickens were ob-
vampire bats. served to suddenly squat down on top of a
80 JOURNAL OF MAMMALOGY Vol. 80, No.1
feeding bat. No ill effects have been ob- between these genera may be reduced
served in bats in those situations, and on through resource partitioning.
most occasions there was no interruption in
ACKNOWLEDGMENTS
feeding.
We believe that the stance of D. youngi This study was made possible by funding
is less upright than that of D. rotundus for from the American Museum of Natural History
two additional reasons: climbing efficiency (Theodore Roosevelt Memorial Fund. Coleman
Postdoctoral Fellowship); the American Society
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
and crypsis. As a climber and arboreal
hunter, D. youngi has decreased the tenden- of Mammalogists (grant-in-aid). Sigma Xi. and
Cornell University. Special thanks to the Min-
cy to topple from its support (the branch)
istry of Agriculture, Lands and Marine Re-
by several means: 1) keeping its center of
sources. Trinidad. The authors thank the follow-
mass close to the support (this is especially ing individuals in Trinidad: the vampire-bat
valauble during vertical climbing behavior); crew (A. Johnson and P. Wallace). D. Booda. C.
2) climbing underneath the branch; 3) using James, S. Johnstone. N. Gyan. H. Nelson, S.
its thumbs and hind feet to exert a torque Ramdass. J. Latchman, Ali family. and especial-
that resists the toppling moment (for a re- ly G. Ramsawak and O. Ramsawak (our hosts
view of the biomechanics of climbing see at Mount Saint Benedict's). We thank individu-
Cartmill, 1985). Additionally, by clinging als at Cornell University: J. W. Hermanson. J.
with its body under and close to the branch, Bertram, D. McCleam. H. Evans. D. Cullinane,
D. youngi is better hidden from the sight of Y. H. Chang. C. Coen. P. Faure. R Quimby. L.
Carbone. H. Bartlett. D. Woodin. C. Grant, and
its avian prey. D. youngi, initiates flight by
L. L. Lafrance. We thank the Department of
simply dropping from its arboreal feeding
Mammalogy at the American Museum of Nat-
position and has no need for the flight-ini- ural History, especially N. B. Simmons, R.
tiating jumps that are characteristic of the MacPhee, D. Lunde. T. Conway. and K. R
terrestrially feeding D. rotundus. Koopman. Thanks also to J. S. Altenbach. R.
In summary, specimens of D. youngi and Dugal. R. Adamo. R. "Tuna" Sinclair. W. Uie-
D. rotundus captured in Trinidad have been da. A. Stem. T. Kunz, M. Schutt. J. Schutt. W.
maintained successfully in captivity. The only R. Schutt. and Bloomfield College (especially J.
difference in maintenance programs for these Noonan. I. Anderson. M. LaBare, and P. Russo).
two genera is a once per week supplement of This paper is dedicated to our friend. A. M.
live chicken blood for D. youngi. Many dif- Greenhall, who pioneered the modem study of
vampire bats. Without his encouragement. this
ferences in behavior, however, have been not-
work would not have been undertaken.
ed between D. youngi and D. rotundus. We
believe that these differences are related to LITERATURE CITED
their mode of prey selection. D. rotundus ex-
ALTENBACH. J. S. 1979. Locomotor morphology of the
hibits a number of unique adaptations for ter- vampire bat. Desmodus rotundus. Special Publica-
restrial feeding (e.g., speed, flight-initiating tion, The American Society of Mammalogists, 6:1-
jumps). Although D. youngi is quite capable 137.
- - - . 1988. Locomotion. pp. 71-83, in Natural his-
of terrestrial feeding, there have been no re- tory of vampire bats (A. M. Greenhall and U.
ports of this mode of feeding in the wild, and Schmidt, eds.). CRC Press, Boca Raton, Florida.
it appears that D. youngi is better adapted for CARTMILL, M. 1985. Climbing. Pp. 73-88, in Func-
tional vertebrate morphology (M. Hildebrand, ed.).
arboreal feeding. Blood feeding in mammals Harvard University Press, Cambridge, Massachu-
is restricted to three genera of vampire bats, setts.
and these exhibit many unique adaptations DICKSON, J. M., AND D. G. GREEN. 1970. The vampire
bat (Desmodus rotundus): improved methods of lab-
that allow them to maintain a sanguivorous oratory care and handling. Laboratory Animals, 4:
lifestyle (e.g., highly modified digestive and 37-44.
excretory systems, quadrupedal locomotion, DISANTO, p. 1960. Anatomy and histochemistry of the
salivary glands of the vampire bat, Desmodus rotun-
altruistic behavior). In Trinidad where D. ro- dus murinus. Journal of Morphology, 106:301-336.
tundus and D. youngi coexist, competition DITMARS, R. L., AND A. M. GREENHALL. 1935. The
1999 SCHUTT ET AL.-DlAEMUS YOUNG1 IN CAPTIVITY 81
vampire bat: a presentation of undescribed habits sities Federation for Animal Welfare handbook on the
and review of its history. Zoologica, 19:53-75. care and management of laboratory animals. Fourth
GARDNER, A. L. 1977. Feeding habits. Pp. 293-350, in ed. Williams and Wilkins Company, Baltimore,
Biology of bats of the New World family Phyllos- Maryland.
tomatidae, Part II (R. J. Baker, J. K. Jones, Jr., and SAZIMA, I. 1978. Aspectos do comportamento alimentar
D. C. Carter, eds.). Special Publications, The Mu- do morcego hematofago, Desmodus rotundus. Bole-
seum, Texas Tech University, 13:1-364. tim de Zoologia, Sao Paulo, 3:97-119.
GOODWIN, G. G., AND A M. GREENHALL. 1961. A re- SAZIMA, I., AND W. UIEDA. 1980. Feeding behavior of
view of the bats of Trinidad and Tobago: descriptions, the white-winged vampire bat, Diaemus youngi, on
rabies infection, and ecology. Bulletin of the Ameri- pOUltry. Journal of Mammalogy, 61:102-104.
Downloaded from https://academic.oup.com/jmammal/article-abstract/80/1/71/844785 by guest on 30 June 2020
can Museum of Natural History, 122:187-301. SCHUrr, W. A, JR., AND J. S. ALTENBACH. 1997. A sixth
GREENHALL, A M. 1965. Notes on behavior of captive digit in Diphylla ecaudata, the hairy legged vampire
vampire bats. Mammalia, 29:441-451. bat. Mammalia, 61:280-285.
- - - . 1976. Care in captivity. pp. 89-131, in Biology SCHUrr, W. A, JR., ET AL. 1997. Functional morphology
of bats of the New World family Phyllostomatidae, of the common vampire bat, Desmodus rotundus. The
Part I (R. J. Baker, J. K. Jones, Jr., and D. C. Carter, Journal of Experimental Biology, 200:3003-3012.
eds.). Special Publications, The Museum, Texas Tech TRAPIDO, H. 1946. Observations on the vampire bat
University, 10:1-218. with special reference to longevity in captivity. Jour-
- - - . 1988. Feeding behavior. pp. 111-131, in Nat- nal of Mammalogy, 27:217-219.
ural history of vampire bats (A M. Greenhall and U. UIEDA, W. 1986. Aspectos da morfologia lingual das
Schmidt, eds.). CRC Press, Boca Raton, Florida. tres especies de morcegos hematofagos (Chiroptera,
GREENHALL, A M., AND U. SCHMIDT. 1988. Natural his- Phyllostomidae). Revista Brasileira de Biologia, 46:
tory of vampire bats (A M. Greenhall and U. 581-587.
Schmidt, eds.). CRC Press, Boca Raton, Florida. UIEDA, W., AND V. F. DE ARAUJO. 1987. Mantencao dos
GREENHALL, A M., AND W. A. SCHUrr, JR. 1996. Diae- morcegos hematofagos Diaemus youngi e Diphylla
mus youngi. Mammalian Species, 533:1-7. ecaudata (Chiroptera, Phyllostomidae), em cativei-
HANDLEY, C. 0., JR. 1976. Mammals of the Smithsonian roo Anais Seminario Ciencias da Fiube, Uberaba, 1:
Venezuelan Project. Brigham Young University Sci- 30-42.
ence Bulletin, Biological Series, 20(5):1-91. UIEDA, W., S. BUCK, AND I. SAZIMA. 1992. Feeding
HAWKEY, C. M. 1966. Plasminogen activator in saliva behavior of the vampire bats, Diaemus youngi and
of the vampire bat Desmodus rotundus. Nature, 211: Diphylla ecaudata, on smaller birds in captivity.
434-435. Ciencia e Cultura, 44:410-412.
- - - . 1967. Inhibitor of platelet aggregation present VILLA-R., B. 1967. Los murcielagos de Mexico: su im-
in saliva of the vampire bat Desmodus rotundus. Brit- portancia en la economfa y la salubridad-su clasi-
ish Journal of Haematology, 13:1014-1020. ficaci6n sistematica. Instituto de Biologfa, Univer-
HoYT, R. A, AND J. S. ALTENBACH. 1981. Observations sidad Nacional Aut6noma de Mexico, Mexico, Dis-
on Diphylla ecaudata. Journal of Mammalogy, 62: trito Federal, Mexico.
215-216. WILKINSON, G. S. 1984. The social organization of the
JOERMANN, G. 1988. Care of vampire bats in captivity. common vampire bat. II. Mating system, genetic
pp. 227-232, in Natural history of vampire bats (A structure and relatedness. Behavioral Ecology and
M. Greenhall and U. Schmidt, eds.). CRC Press, Boca Sociobiology, 17: 123-129.
Raton, Florida. - - - . 1988. Social organization and behavior. pp
KOOPMAN, K. F. 1988. Systematics and distribution. pp. 85-97, in Natural history of vampire bats (A M.
7-17, in Natural history of vampire bats (A M. Greenhall and U. Schmidt, eds.). CRC Press, Boca
Greenhall and U. Schmidt, eds.). CRC Press, Boca Raton, Florida.
Raton, Florida. WIMSATT, W. A I 962a. Observations on the feeding
KUNZ, T. H., AND E. ANTHONY. 1982. Age estimation capacities and excretory functions of captive vam-
and post-natal growth in the bat, Myotis lucifugus. pire bats. Journal of Mammalogy, 43:17-27.
Journal of Mammalogy, 63:23-32. - - - . 1962b. Responses of captive common vam-
MENDEZ, E. 1988. Parasites of vampire bats. pp. 191- pires to cold and warm environments. Journal of
214, in Natural history of vampire bats (A M. Green- Mammalogy, 43:185-191.
hall and U. Schmidt, eds.). CRC Press, Boca Raton, - - - . 1978. Vampire bats. Pp. 507-513, in Zoo and
Florida. wild animal medicine (M. E. Fowler, ed.). Saunders
MURADAU, F., N. MONOOL, AND W. A SCHUrr, JR. 1993. Press, Philadelphia, Pennsylvania.
WIMSATT, W. A., AND A. GUERRIERE. 1961. The main-
Observations on feeding behavior in the white-
tenance of the common vampire Desmodus rotundus
winged vampire bat, Diaemus youngi. Bat Research
murinus in captivity. Journal of Mammalogy, 42:
News, 34:121A
449-455.
NOVICK, A 1963. Orientation in Neotropical bats. II.
WIMSATT, W. A, A GUERRIERE, AND R. HORST. 1973.
Phyllostomatidae and Desmodontidae. Journal of
An improved cage design for maintaining vampires
Mammalogy, 44:44-56. (Desmodus) and other bats for experimental purpos-
PYE, J. D. 1967. Bats. pp. 491-501, in The Universities es. Journal of Mammalogy, 54:251-254.
Federation for Animal Welfare handbook on care and
management of laboratory animals. Third ed. Living- Submitted 19 August 1997. Accepted 16 June 1998.
stone, Edinburgh, United Kingdom.
RACEY, P. A 1972. Bats. pp. 296-308, in The Univer- Associate Editor was Troy L. Best.
You might also like
- Assam Tribune and Sentinel Editorials From Jan To Oct 31 2023Document726 pagesAssam Tribune and Sentinel Editorials From Jan To Oct 31 2023Simi GogoiNo ratings yet
- Test Bank For Experiencing Mis 8th by KroenkeDocument36 pagesTest Bank For Experiencing Mis 8th by Kroenkeclavisgowdenet28100% (34)
- Dog Boarding ContractDocument3 pagesDog Boarding ContractIulik Bruma100% (1)
- How To Draw Manga Sketching (Manga-Style) - Vol. 2 Logical ProportionsDocument182 pagesHow To Draw Manga Sketching (Manga-Style) - Vol. 2 Logical Proportionsrichie89% (18)
- Capturing and Handling Wild Animals PDFDocument55 pagesCapturing and Handling Wild Animals PDFscrane@100% (1)
- Multiple Pregnancy LectureDocument33 pagesMultiple Pregnancy Lecturefarid_nchep100% (1)
- Observations of The Common Vampire Bat (Desmodus Rotundus) and The Hairy-Legged Vampire Bat (Diphylla Ecaudata) in CaptivityDocument14 pagesObservations of The Common Vampire Bat (Desmodus Rotundus) and The Hairy-Legged Vampire Bat (Diphylla Ecaudata) in CaptivityNissa SissariNo ratings yet
- CONICET Digital Nro. ADocument1 pageCONICET Digital Nro. ADescargaryaNo ratings yet
- Tree Use by HarvestmenDocument6 pagesTree Use by Harvestmenleilany casillasNo ratings yet
- Vampire BatsDocument2 pagesVampire BatsMaíra Sant'AnaNo ratings yet
- Herpetological Bulletin: Number 106 - Winter 2008Document45 pagesHerpetological Bulletin: Number 106 - Winter 2008Umair AneesNo ratings yet
- Diet and Feeding Behaviour of Snapping Turtles (Chelydra Serpentina) and Midland Painted Turtles (Chrysemys Picta Marginata) in Algonquin Provinc...Document7 pagesDiet and Feeding Behaviour of Snapping Turtles (Chelydra Serpentina) and Midland Painted Turtles (Chrysemys Picta Marginata) in Algonquin Provinc...blackriptoniteNo ratings yet
- Sibonius Is A Diurnal, Actively Foraging Snake Inhabiting TheDocument2 pagesSibonius Is A Diurnal, Actively Foraging Snake Inhabiting Thelaspiur22blues7327No ratings yet
- Ejemplo de Una Nota InvestigativaDocument2 pagesEjemplo de Una Nota InvestigativaJayRo HdezNo ratings yet
- Taxonomy of Indonesian Giant Clams (Cardiidae, Tridacninae) : Udhi Eko HernawanDocument6 pagesTaxonomy of Indonesian Giant Clams (Cardiidae, Tridacninae) : Udhi Eko Hernawannugraha95No ratings yet
- 1 - Finches on Islands: Đề Cương Ôn Tập Reading 2022 Ielts Đình LongDocument16 pages1 - Finches on Islands: Đề Cương Ôn Tập Reading 2022 Ielts Đình LongVũ Thùy Bảo TrâmNo ratings yet
- Biology Lab 2Document2 pagesBiology Lab 2Lauren ShawNo ratings yet
- Bonaccorso Et Al. 2002. Home Range of Dobsonia Minor PDFDocument10 pagesBonaccorso Et Al. 2002. Home Range of Dobsonia Minor PDFHenry CondoriNo ratings yet
- Art 14Document4 pagesArt 14Claudio Campeador SanhuezaNo ratings yet
- A New Species of Gekkonid Lizard (Sphaerodactylinae: Gonatodes) From Guyana, South AmericaDocument16 pagesA New Species of Gekkonid Lizard (Sphaerodactylinae: Gonatodes) From Guyana, South AmericaRenata PerezNo ratings yet
- Dicamptodon Ensatus: Predation by Thamnophis Couchii OnDocument3 pagesDicamptodon Ensatus: Predation by Thamnophis Couchii OnJulio Acuña VNo ratings yet
- Div Class Title Evaluating Mortality Sources For The Vulnerable Pudu Span Class Italic Pudu Puda Span in Chile ImplicationsDocument7 pagesDiv Class Title Evaluating Mortality Sources For The Vulnerable Pudu Span Class Italic Pudu Puda Span in Chile ImplicationsClaudio Campeador SanhuezaNo ratings yet
- Hippocampus and Amygdala. Bachara 1995Document5 pagesHippocampus and Amygdala. Bachara 1995David LopezNo ratings yet
- AMRE2417Document9 pagesAMRE2417tortugamarinaNo ratings yet
- Trapping The Oldest ProfessionDocument9 pagesTrapping The Oldest ProfessiontLzmS23No ratings yet
- Yap Et Al 2023 Taxonomy and Molecular Phylogeny of The Sea Anemone Macrodactyla With A Description of A New Species From SingaporeDocument25 pagesYap Et Al 2023 Taxonomy and Molecular Phylogeny of The Sea Anemone Macrodactyla With A Description of A New Species From SingaporenickYNo ratings yet
- The Behavior of The Bush Dog (Speothos Venaticus Lund, 1842) in The Field - A ReviewDocument7 pagesThe Behavior of The Bush Dog (Speothos Venaticus Lund, 1842) in The Field - A Reviewcamylle carvalhoNo ratings yet
- Mehitzoth AnalysisDocument2 pagesMehitzoth AnalysisKatherin LuceroNo ratings yet
- Woodward Et Al 2019 - Characteristic of American Alligator Bites On People in FloridaDocument17 pagesWoodward Et Al 2019 - Characteristic of American Alligator Bites On People in FloridaGabriel NguyenNo ratings yet
- Effects of Parasites in Marine MammalsDocument8 pagesEffects of Parasites in Marine MammalsRaúl GDNo ratings yet
- Brownetal JSR 2002 SemeleyGariDocument9 pagesBrownetal JSR 2002 SemeleyGariMarco Antonio SolisNo ratings yet
- Kahl Et Al., 2012 PDFDocument23 pagesKahl Et Al., 2012 PDFNo NameNo ratings yet
- Squirrels As PredatorsDocument9 pagesSquirrels As PredatorsfsurferNo ratings yet
- Systematics and Conservation of The Hook-Billed KiDocument12 pagesSystematics and Conservation of The Hook-Billed Ki20 074 NGAKAN NYOMAN SUARTANo ratings yet
- Henderson 2009Document25 pagesHenderson 2009Karla MorenoNo ratings yet
- Astié (2003) - New Record of Shiny Cowbird (Molothrus Bonariensis) Parasitism of Black-Chinned Siskins (Carduelis Barbata)Document10 pagesAstié (2003) - New Record of Shiny Cowbird (Molothrus Bonariensis) Parasitism of Black-Chinned Siskins (Carduelis Barbata)Paola ResendeNo ratings yet
- 058 009 0101 PDFDocument19 pages058 009 0101 PDFwilllNo ratings yet
- American Society of Ichthyologists and Herpetologists (ASIH), Allen Press CopeiaDocument5 pagesAmerican Society of Ichthyologists and Herpetologists (ASIH), Allen Press CopeiaDébora Samira G. NegrãoNo ratings yet
- TH TH: R e C I B I D O: 0 1 / 0 3 / 2 0 0 9 - A C e P T A D O: 1 2 / 1 1 / 0 9 Ed. Asoc.: F. CruzDocument4 pagesTH TH: R e C I B I D O: 0 1 / 0 3 / 2 0 0 9 - A C e P T A D O: 1 2 / 1 1 / 0 9 Ed. Asoc.: F. CruzasociacionherpetologicaNo ratings yet
- The Giant Clam Culture Project in The PhilippinesDocument3 pagesThe Giant Clam Culture Project in The PhilippinesCraige SamNo ratings yet
- BROWN D B CAMPOS & HJ URBAN 2002 Reproductive Cycle of The Bivalve Clams Semele Solida and Gari Solida From ChileDocument8 pagesBROWN D B CAMPOS & HJ URBAN 2002 Reproductive Cycle of The Bivalve Clams Semele Solida and Gari Solida From ChiledietrichNo ratings yet
- Edmonds 2021 Poison Frogs Traded in US Herp ReviewDocument9 pagesEdmonds 2021 Poison Frogs Traded in US Herp ReviewStan AlcornNo ratings yet
- Morfología de Los Estados Inmaduros y Biología de Cactoblastis Doddi (Lepidoptera: Pyralidae) en La Prepuna de Jujuy (Noroeste de Argentina)Document10 pagesMorfología de Los Estados Inmaduros y Biología de Cactoblastis Doddi (Lepidoptera: Pyralidae) en La Prepuna de Jujuy (Noroeste de Argentina)Gra GomezNo ratings yet
- Emparanza Et Al. 2011 MBRDocument3 pagesEmparanza Et Al. 2011 MBRrulloa1971No ratings yet
- 880 - 28042019 - Baptista - Et - Al - Leptopogon - Amaurocephalus - Eriophora - Fulig Inea - FinalDocument3 pages880 - 28042019 - Baptista - Et - Al - Leptopogon - Amaurocephalus - Eriophora - Fulig Inea - FinalGabriel AssunçãoNo ratings yet
- Shafer 2001 Inter-Reserve DistanceDocument13 pagesShafer 2001 Inter-Reserve DistancealiceNo ratings yet
- Pertumbuhan KecoaDocument6 pagesPertumbuhan KecoaGavin Angga PutraNo ratings yet
- 2 Mora y Robinson Loxocemus - LepidochelysDocument2 pages2 Mora y Robinson Loxocemus - LepidochelysJosé MoraNo ratings yet
- Jee106 2428Document5 pagesJee106 2428A. BNo ratings yet
- WaiteDocument2 pagesWaiteTra TopoNo ratings yet
- A Review of the Middle American Tree Frogs of the Genus PtychohylaFrom EverandA Review of the Middle American Tree Frogs of the Genus PtychohylaNo ratings yet
- Notas de Alimentacion de Micrurus DiastemaDocument6 pagesNotas de Alimentacion de Micrurus DiastemaJosue Ramos GaldamezNo ratings yet
- Dillehay 2008Document4 pagesDillehay 2008erkanaydoganogluNo ratings yet
- Currylowetal 2015bDocument4 pagesCurrylowetal 2015bvenicaveniceNo ratings yet
- Proceedings of the Zoological Institute RAS Vol. 318, No. 2, 2014, рр. 168-176Document9 pagesProceedings of the Zoological Institute RAS Vol. 318, No. 2, 2014, рр. 168-176lacosNo ratings yet
- Case 1994Document15 pagesCase 1994ArvanNo ratings yet
- Istory of Jellyfish Envenomation: 3.1.1. Class Cubozoa - Cubozoan JellyfishDocument16 pagesIstory of Jellyfish Envenomation: 3.1.1. Class Cubozoa - Cubozoan JellyfishRangsiyo BeabeoNo ratings yet
- Kajinetal 2008Document9 pagesKajinetal 2008Raymundo Lopez NNo ratings yet
- Taucareetal Steatoda 2016Document1 pageTaucareetal Steatoda 2016Nacho Delgado FerreiroNo ratings yet
- Nash Et Al 2015Document5 pagesNash Et Al 2015Camila RodríguezNo ratings yet
- 0090-3558-45 4 1235Document5 pages0090-3558-45 4 1235Angel AcostaNo ratings yet
- Pudu PuduDocument8 pagesPudu PuduJose Roberto Vazquez AvendañoNo ratings yet
- Common Birds of Andaman Islands With Special Reference To Introduced BirdsDocument5 pagesCommon Birds of Andaman Islands With Special Reference To Introduced BirdsMonika MohanNo ratings yet
- Roosting Behaviour and Habitat Selection of Pteropus Giganteus Reveal Potential Links To Nipah Virus EpidemiologyDocument12 pagesRoosting Behaviour and Habitat Selection of Pteropus Giganteus Reveal Potential Links To Nipah Virus EpidemiologyIslam Ausraf RajibNo ratings yet
- Review Article: Tracing The SARS-coronavirusDocument4 pagesReview Article: Tracing The SARS-coronavirusIslam Ausraf RajibNo ratings yet
- Behavioral Studies of Bats in Captivity: Methodology, Training, and Experimental DesignDocument20 pagesBehavioral Studies of Bats in Captivity: Methodology, Training, and Experimental DesignIslam Ausraf RajibNo ratings yet
- 0090-3558-45 4 1030 PDFDocument12 pages0090-3558-45 4 1030 PDFIslam Ausraf RajibNo ratings yet
- Sampling Blood From Big Brown Bats (Eptesicus Fuscus) in The Field With and Without Anesthesia: Impacts On SurvivalDocument5 pagesSampling Blood From Big Brown Bats (Eptesicus Fuscus) in The Field With and Without Anesthesia: Impacts On SurvivalIslam Ausraf RajibNo ratings yet
- Anesthesia and Blood Sampling of Wild Big Brown Bats (Eptesicus Fuscus) With An Assessment of Impacts On SurvivalDocument10 pagesAnesthesia and Blood Sampling of Wild Big Brown Bats (Eptesicus Fuscus) With An Assessment of Impacts On SurvivalIslam Ausraf RajibNo ratings yet
- Review of Bats and SARS: Lin-Fa Wang, Zhengli Shi, Shuyi Zhang, Hume Field, Peter Daszak,# and Bryan T. EatonDocument7 pagesReview of Bats and SARS: Lin-Fa Wang, Zhengli Shi, Shuyi Zhang, Hume Field, Peter Daszak,# and Bryan T. EatonIslam Ausraf RajibNo ratings yet
- Biosafety and Health: SciencedirectDocument7 pagesBiosafety and Health: SciencedirectIslam Ausraf RajibNo ratings yet
- Status and Distribution of Bats in Bangladesh With Notes On Their EcologyDocument5 pagesStatus and Distribution of Bats in Bangladesh With Notes On Their EcologyIslam Ausraf RajibNo ratings yet
- 120 Adult Party GamesDocument24 pages120 Adult Party Gamesbhuvnesh.bhatt100% (1)
- Blood Group LabDocument12 pagesBlood Group Labvinayakjeet03No ratings yet
- Digestive SystemDocument18 pagesDigestive SystemSwit Manrique-De HittaNo ratings yet
- Frequency of Genotypic MarkersDocument12 pagesFrequency of Genotypic MarkersZharick Juliana Perdomo FandiñoNo ratings yet
- Cross Sectional Anatomy of SpineDocument37 pagesCross Sectional Anatomy of SpineAvinash Gupta50% (2)
- The Harpy Eagle (Harpia Harpyja) in The Infierno Native CommunityDocument18 pagesThe Harpy Eagle (Harpia Harpyja) in The Infierno Native CommunityJeff CremerNo ratings yet
- Adoption Agreement Release ContractDocument2 pagesAdoption Agreement Release ContractgumNo ratings yet
- 3phonics Reading Short Long DiptongsDocument62 pages3phonics Reading Short Long DiptongsMike Lee100% (2)
- March 2016 MailerDocument2 pagesMarch 2016 Mailerapi-208882322No ratings yet
- Masterfood DMADocument7 pagesMasterfood DMAPatricia HNo ratings yet
- WonderlandDocument2 pagesWonderlandJohn WilliamsNo ratings yet
- Tooth TroubleDocument35 pagesTooth TroubleReegen TeeNo ratings yet
- The Question of Humanity in Gulliver's Travels Book IVDocument11 pagesThe Question of Humanity in Gulliver's Travels Book IVKristine Reynaldo100% (1)
- Somiya Mehta CorporateDocument2 pagesSomiya Mehta Corporateapi-345724380No ratings yet
- TUGAS EXERCISE Counjantion 5,7, DAN SepuluhDocument3 pagesTUGAS EXERCISE Counjantion 5,7, DAN SepuluhYunizar LidyaNo ratings yet
- Bears Everywhere - Songs Poems and Fingerplays PDFDocument30 pagesBears Everywhere - Songs Poems and Fingerplays PDFLourdes Gimenez NaveaNo ratings yet
- Backcross Breed by ChimeraDocument4 pagesBackcross Breed by Chimerayog sothothNo ratings yet
- Fauna Study MarrickvilleDocument72 pagesFauna Study MarrickvilleHelayne Short0% (1)
- Recount TextDocument14 pagesRecount TextindahpriliatyNo ratings yet
- Biomechanics NotesDocument35 pagesBiomechanics NotesSunitha JosephineNo ratings yet
- Fey DragonDocument5 pagesFey DragonBigMekUghNo ratings yet
- Save These Instructions!: Retro Hot Dog Roller ™Document18 pagesSave These Instructions!: Retro Hot Dog Roller ™Danielle HallNo ratings yet
- Guardian Animal CrossingsDocument6 pagesGuardian Animal CrossingsPaulNo ratings yet
- Mind Mapping! Group: .: Some Sentences Based OnDocument3 pagesMind Mapping! Group: .: Some Sentences Based OnAntonius GusiNo ratings yet
- William Golding: Writing SuccessDocument3 pagesWilliam Golding: Writing SuccessBrezoi OanaNo ratings yet