Professional Documents
Culture Documents
Neurophysiology
Neurophysiology
Uploaded by
Rachel Cajiles0 ratings0% found this document useful (0 votes)
51 views117 pagesThis document summarizes key concepts in neurophysiology including biological membranes, ion channels, ion pumps, membrane potential, and action potentials. Biological membranes are lipid bilayers that enclose cells and organelles. Embedded in the bilayer are protein channels and pumps that regulate the flow of ions across membranes. Ion channels like voltage-gated sodium channels allow selective ion flow. Ion pumps like the sodium-potassium pump actively transport ions against their gradients. The distribution of ions creates a membrane potential, with the inside negatively charged at rest. Local potentials like synaptic potentials can cause graded depolarization, while an action potential is an all-or-none spike triggered when the threshold is reached.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes key concepts in neurophysiology including biological membranes, ion channels, ion pumps, membrane potential, and action potentials. Biological membranes are lipid bilayers that enclose cells and organelles. Embedded in the bilayer are protein channels and pumps that regulate the flow of ions across membranes. Ion channels like voltage-gated sodium channels allow selective ion flow. Ion pumps like the sodium-potassium pump actively transport ions against their gradients. The distribution of ions creates a membrane potential, with the inside negatively charged at rest. Local potentials like synaptic potentials can cause graded depolarization, while an action potential is an all-or-none spike triggered when the threshold is reached.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
51 views117 pagesNeurophysiology
Neurophysiology
Uploaded by
Rachel CajilesThis document summarizes key concepts in neurophysiology including biological membranes, ion channels, ion pumps, membrane potential, and action potentials. Biological membranes are lipid bilayers that enclose cells and organelles. Embedded in the bilayer are protein channels and pumps that regulate the flow of ions across membranes. Ion channels like voltage-gated sodium channels allow selective ion flow. Ion pumps like the sodium-potassium pump actively transport ions against their gradients. The distribution of ions creates a membrane potential, with the inside negatively charged at rest. Local potentials like synaptic potentials can cause graded depolarization, while an action potential is an all-or-none spike triggered when the threshold is reached.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 117
Neurophysiology
Biological membranes, synapses, neurotransmitters, electrical and
chemical bases of nerve function
Biological Membranes
• They are the outer covering of cells which is also called Plasma Membrane.
• They enclose all cells in the body as well as the organelles within these
cells.
• Biological membrane or plasma membrane is a lipid bilayer with the polar
(hydrophilic) heads facing outward and the nonpolar (hydrophobic) tails
extending to the middle of the bilayer. Embedded in this bilayer are protein
macromolecules including ion channels, receptors, and ionic pumps that
are in contact with both the extracellular fluid and the cytoplasm.
• The lipid bilayer is relatively impermeable to water soluble molecules
including ions like Na+, K+, Cl-, and Ca++.
• The concentrations of Na+, Cl-, and Ca++ are higher extracellularly, while
K+ and anionic proteins are higher intracellularly.
Biological Membrane
Ion Channels
• Ion channels are transmembrane proteins that form hydrophilic pores
through the lipid bilayer.
• They allow the passive flow of selected ions across the membrane on
the basis of electrochemical gradients of the ion and the physical
properties of the ion channel.
• The amino acid composition of the channel subunits forming the
hydrophilic pores determines the ionic selectivity of the channel.
Types of ion channels
• Most ion channels are gated meaning, they open in response to
specific stimuli. Based on this, ion channels can be divided into :
1. Voltage gated ion channels which respond to changes in membrane
potential.
Ex. Voltage gated Na+ channels, K+, Ca++ which control almost all the
signals for rapid communication in the nervous system.
2. Ligand (Neurotransmitter) gated ion channels which respond to the
binding of a neurotransmitter to the channel molecule complex.
Ex. Acetylcholine receptor, glutamate receptor.
General structure of a voltage gated cation
channel
• Voltage gated cation channel consists of pore-forming subunits called alpha
subunits and a variable number of auxiliary subunits (beta, gamma, epsilon).
• The alpha subunits determine ion selectivity, mediate the voltage sensing of the
channel and are sufficient for the function of the channel.
• Example : voltage gated K+ channels are made up of 4 homologous alpha
subunits, each consisting of a polypeptide with 6 helical transmembrane
segments (S1-S6) linked by intracellular and extracellular loops; its N and C
terminal regions face the cytoplasm. The S4 segment acts as the voltage sensor,
and the P loop located between the S5 and S6 helices of each domain forms the
mouth of the pore and acts as a selectivity filter, regulating ion permeability. The
auxiliary subunits profoundly affect the time course and voltage dependence of
channel activation or inactivation and influence the assembly and expression of
voltage gated channel subunits.
Ion Pumps
• Ion pumps are also transmembrane proteins that transport ions across the
membrane against their concentration gradient with the consumption of
ATP which is provided by oxidative metabolism of glucose (aerobic
glycolysis) involving the Kreb’s cycle and respiratory chain in mitochondria.
• The ion pump is important for maintenance of transmembrane ion
concentration gradient.
• Example of ion pump : Na+K+ ATPase pump. As there is continuous leakage
of K+ out of the cell and of Na+ into the cell driven by both the
concentration gradient and electrical gradient, the transmembrane ion
gradient is restored by the Na+K+ATPase pumps which pump 3Na+ out for
every 2 K+ in.
Membrane Potential
• Membrane potential is the electrical potential generated by the
electrical activity of a cell. It is the difference in the electrical potential
between the inside and outside of a cell.
• Determinants of Membrane potential :
1.Transmembrane ion concentration gradient
2.Permeability of the membrane to each ion
• It includes resting membrane potential, local potential and action
potential
Ionic mechanisms of membrane potential
• Ions are distributed unequally on the inside and outside of cells and that cell
membranes are selectively permeable to different ions. K+ is higher inside than
outside so K+ will move from inside to outside making the inside more negatively
charge than outside. This movement of K+ outward is called the diffusion
pressure. To balance between this difference in charges, the negatively charged
ions (Proteins) will try to pull the K+ inward and this voltage produces electrical
pressure that opposes the movement of K+. Eventually an equilibrium will be
established. This is the equilibrium potential of ions or the Nernst Equilibrium
Potential.
• Equilibrium potential is the electrical potential that develops across the
membrane at equilibrium. It is the voltage difference across the membrane that
exactly offsets the tendency of the ion to move down its concentration gradient.
• The transmembrane concentration gradient and the charge of each ion (voltage)
determine the equilibrium potential of that ion.
Ionic mechanisms of membrane potentials
• Based on the diffusion pressure and electrical pressure, the Nernst
equation is used to define the equilibrium potential inside the cell for
any ions in terms of its concentrations on the two sides of a
membrane.
• Electrical pressure is We = Em x Zi x F where
We = electrical pressure (work required to move an ion against a
voltage).
Em = absolute membrane potential
Zi = valence (number of charges on the ion)
F = Faraday
Ionic mechanisms of membrane potential
• Diffusion pressure is Wd = R x T x In(Chi – Clo) where
Wd = diffusion pressure (work required to move an ion against a
concentration gradient)
R = universal gas constant
T = absolute temperature
In = natural logarithm
Chi = ion concentration on higher side
Clo = ion concentration of lower side
Ionic mechanisms of membrane potential
• At equilibrium , We = Wd therefore
Em x Zi x F = RT x In (Chi – Clo)
By rearrangement, the equilibrium potential is
Em = RT/FZi x In (Chi – Clo)
By substituting for the constants at RT, converting to a base 10 logarithm
and converting to mV, a useful equation is obtained : Nernst Equation
Em = 58 log10 Chi/Clo
Example E(Na) = 58 log10 140/25
= 43.3 mV (Equilibrium potential of Na+)
Ionic mechanisms of membrane potential
• Julius Bernstein in 1900 suggested that the resting membrane
potential was equal to the equilibrium potential of K+.
• An experiment to test Bernstein hypothesis is illustrated.
Ionic mechanisms of membrane potential
• Goldman-Hodgkin and Katz (GHK) equation : when a membrane is
permeable to 2 different ions, the Nernst equation can no longer be
used to precisely determine the membrane potential. The GHK
equation is recommended.
R ∙T PK ∙ K + O + PNa ∙ Na+ O + PCl ∙ Cl− i
Em = ∙ ln
F ∙Z PK ∙ K + i + PNa ∙ Na+ i + PCl ∙ Cl− o
Resting membrane potential
• The resting membrane potential (RMP) is the absolute difference in
electrical potential between the inside and outside of an inactive
neuron, axon or muscle cell. It is the transmembrane voltage at which
there is no net flow of current across the membrane. The RMP is
generally between 60-80 mV with the inside of the cell negative.
• A decrease in the value of RMP means less negative inside of the cell
and the membrane potential moves toward Zero and cell is more
excitable This constitutes depolarization.
• When the RMP becomes more negative, the potential moves away
from Zero and cell is less excitable. This is hyperpolarization.
Resting membrane potential
• RMP depends on 2 factors :
1.Presence of leak ion channels open at rest with markedly different permeabilities
to K+ making the cell membrane a semi-permeable membrane.
2.Presence of energy dependent pumps eg. Na+K+ pump.
At rest, there is a continuous leak of K+ outward and of Na+ inward across the
membrane. The Na+K+ pump maintains the intracellular concentrations of Na+ and
K+ despite their constant leaking through the cell membrane by transporting 3 Na+
out for every 2 K+ in. This leads to a state in which the net movement of ion across
the membrane is zero. This is called steady state and this constitutes the RMP.
The cell at rest is also permeable to Cl- ions. In most membranes, Cl- reaches
equilibrium simply by adjustment of its internal concentration to maintain
electroneutrality without affecting the steady state.
Local potentials
• A local potential is a transient depolarizing or hyperpolarizing shift of the
membrane potential in a localized area of the cell.
• Local potentials result from current flow due to localized change in ion
channel permeability to one or more ions.
• A local potential can be a
1. Synaptic potential : produced by chemical neurotransmitter acting on the
synapse. Ex. Ach produces a fast excitatory postsynaptic potential. GABA
produces a slow inhibitory postsynaptic potential.
2. Receptor potential : produced by activation of a sensory receptor channel
by a stimulus. Ex. Pain, temperature, touch,
3. Electrotonic potential : produced from an externally applied voltage.
Local potentials
• Local potential is a graded response proportional to the size of the
stimulus. The occurrence of a second stimulus before the first one
subsides results in a larger local potential. Therefore, local potentials
can be summated. It can either be temporal summation or spatial
summation.
• Temporal summation is the summation of local potentials from
repeated inputs at the same site but on a different time.
• Spatial summation is the summation of local potentials from multiple
sites of inputs but at the same time.
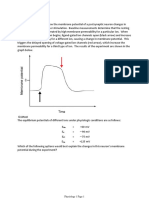
Action potential
• The excitability of a neuron, axon, or muscle cell is defined as the
probability that the neuron or muscle cell will generate or transmit an
action potential. Triggering of an action potential depends on the opening
of voltage gated Na+ channel. The membrane needs to reach a value that
activates the channel. This is called threshold. For a neuron with RMP of -
60-80 mV, the threshold is approximately 10-15 mV from the RMP (-55 mV)
• During stimulation, when threshold is reach, the voltage gated Na+
channels open allowing Na+ to enter the cell making the membrane
potential toward the equilibrium potential of Na which is +40 mV. This is
depolarization. In most cases, this is transient lasting only a few seconds so
that the Na+ channel closes rapidly. At the same time, there is opening of
slowly activating K+ channels allowing K+ to go out of the cell shifting the
membrane potential to the equilibrium potential of K+ which is -100 mV.
This is repolarization.
Synaptic transmission
• A synapse is a specialized contact zone where one neuron
communicates with another neuron.
• There are 2 types of synapses : chemical and electrical.
• Chemical synapses are the more common form of communication in
the nervous system. A chemical synapse consists of
1. pre-synaptic component (containing the synaptic vesicles).
2. Postsynaptic component (dendrite, soma or axon).
3. An intervening space called synaptic cleft.
They make use of chemical messengers called “neurotransmitters” for
communication.
Synaptic transmission
• Rapid diffusion of a chemical messenger across the synaptic cleft is
followed by binding of this substance to receptors spanning the
postsynaptic membrane. There is a resultant alteration in the
electrical, biochemical or genetic properties of that neuron.
• These chemical messengers or neurotransmitters are released in
discrete units called “quanta” from pre-synaptic axonic or dendritic
terminals in response to depolarization of the terminal.
Neurochemical transmitters
I. Amino Acids III. Acetycholine VII. Neurotrophc factors
1. L glutamate IV. Nucleosides/nucleotides 1. nerve growth factor or
2. Aspartate 1. adenosine neurotrophins
3. GABA 2. ATP V. Neuropeptides a. brain derived growth factor
4. Glycine V. Neuropeptides b. neurotrophin 3 (NT3)
II. Monoamines 1. Neurotensin c. neurotrophin 4/5 (NT4/5)
1. Catecholamines 2. Neurokinin (Substance P) 2. glial derived growth factor
a. Dopamine 3. VIP
b. Norepinephrine 4. CGRP
c. Epinephrine VI. Nitric oxide
2. Serotonin
3. Histamine
Criteria necessary to define a substance as a
Neurotransmitter
• Localization : a neurotransmitter must be localized to the presynaptic
elements of an identified synapse and must also be present within
the neuron from which the presynaptic terminal arises.
• Release : the substance must be shown to be released from the
presynaptic element on activation of that terminal and
simultaneously with depolarization of the parent neurons.
• Identity : application of the putative neurotransmitter to the target
cells must be shown to produce the same effects as those produced
by stimulation of the neurons in question.
Neurotransmitters can be categorized as
• Small molecule messengers (having less than 10 carbon atoms)
1.Amino acids : GABA, glycine, aspartate, glutamate
2.Biogenic amines : acetylcholine, catecholamines, serotonin, histamine
3.Nucleotides or nucleosides : adenosine, ATP
4.Others : nitric oxide
• Large molecule messengers (having more than 10 carbon atoms) :
neuropeptides
1.Methionine enkephalins 4. calcitonin gene-related peptide (CGRP)
2.Leucine enkephalins 5. arginine vasopressin
3.Endorphins 6. cholecystokinin
Pre-synaptic events
1. The synthesis and vesicular storage of the neurotransmitter.
2. Mobilizing, docking and priming of the synaptic vesicles in the pre-
synaptic terminals.
3. Ca++ dependent neurotransmitter release by exocytosis.
4. Endocytotic recycling of synaptic vesicles.
5. pre-synaptic reuptake and inactivation of the neurotransmitter.
Pre-synaptic events - Synthesis of
neurotransmitter
• Amino aid neurotransmitters (like glutamate, GABA and glycine) and
acetylcholine are synthesized from intermediates of the Kreb’s cycle.
• The monoamines (dopamine, NE, serotonin and histamines) are all derived
from essential amino acid precursors provided by diet. Their synthesis
involves a specific rate limiting enzymatic step that is regulated by the state
of enzymes phosphorylation and feedback mechanisms.
• Amino acid, Ach and monoamine neurotransmitters are incorporated into
synaptic vesicles at the level of the presynaptic terminal.
• Neuropeptides are synthesized in the cell body as a large precursor that
undergoes cleavage and posttranslational modifications as it travels
through the secretory granule pathway and they reach the synaptic
terminal by fast anterograde transport.
Neurotransmitters are stored in 3 types of
vesicles
1.Small vesicles called synaptic vesicles : 50 mm in diameter, appear
clear and empty in electron microscope. They contain GABA,
glutamate and Ach. Another type of small vesicles but electron dense
cores contain the catecholamines.
2.Larger dense core vesicles : 75-150 mm in diameter, less numerous,
contain neuropeptides.
3.Neurosecretory vesicles : 150-200 mm in diameter, contain in
hypothalamic nuclei, contain neurohormones which are especially
concentrated in axon terminals of the hypophysis (posterior
pituitary).
Synaptic vesicles
• Synaptic vesicles are formed initially by budding from the Golgi complex.
After transport to and release from the presynaptic terminal, the
lipoprotein membrane components of the synaptic vesicles are recycled in
the terminals.
• Synaptic vesicle neurotransmitters are synthesized in the axon terminals
hence can be recycled and refilled in the axon terminal. Ex. Ach is
synthesized in the cytosol of the axon and axon terminal and then
transported into synaptic vesicles. Others are synthesized in the vesicle
itself like NE. Its precursor, dopamine is transported into synaptic vesicles
by vesicular monoamine transporter. Dopamine is then converted to NE by
enzyme dopamine B-hydroxylase which is attached to the luminal border of
the vesicular membrane.
Vesicular transporters
• The storage of neurotransmitters in the synaptic vesicles depends on specific
vesicular transporters. There are at least 3 families of intracellular synaptic vesicle
transporter
1.SLC8 gene family
a. VMAT : Vesicular monoamine transporter (serotonin, NE, DA, histamine)
b. VachT : Vesicular Ach transporter
2. SLC32 gene family : VIAAT Vesicular inhibitory AA transporter (GABA, Glycine)
3. SLC17 gene family : VgluT Vesicular glutamate transporter
Vesicular storage of neurotransmitter is driven by an electrochemical gradient of H+
across the vesicle membrane. This gradient is generated by the vesicular ATP
dependent proton pump. Within the vesicle, neurotransmitter may be co-stored
with ATP, synthetic enzymes or ions like Zn++.
Pre-synaptic events - Exocytosis
• After a synaptic vesicle is loaded with neurotransmitter, it undergoes
mobilization and docking at a presynaptic active zone, followed by
priming for Ca++ triggered exocytosis.
• The synaptic vesicles that are ready for release dock near the
presynaptic active zones, which contain clusters of voltage gated Ca++
channels. Exocytosis is triggered by depolarization of the presynaptic
terminals which allows a massive and transient Ca++ influx through
voltage gated Ca++ channels in response to each action potential.
• After the synaptic vesicles fuse with the synaptic membrane and
release the neurotransmitter, the vesicular membrane proteins are
retrieved by endocytosis and recycled.
Pre-synaptic events – Neurotransmitter
release, presynaptic membrane proteins
All these processes involve complex interactions between synaptic
vesicle and presynaptic membrane proteins. The synaptic vesicle
synapsin links the vesicle to the cytoskeleton and undergoes
phosphorylation to facilitate vesicle mobilization
Membrane docking, priming and fusion depend on interactions of
synaptobrevin located in the synaptic vesicle and 2 proteins, syntaxin
and SNAP-25 located in the presynaptic membrane.
These 3 proteins are the targets of cleavage of botulinum toxins which
impair neurotransmitter release.
Presynaptic events – Neurotransmitter
release, presynaptic membrane proteins
• Calcium induced exocytosis requires interactions with the synaptic
vesicle protein synaptotagmin.
• Vesicle endocytosis and recycling involve vesicle coating by clathrin
and fusion by dynamin. These processes are regulated by adaptor
proteins such as amphiphysin.
• The presynaptic voltage gated Ca++ channels are the N and P/Q type
channels which form complexes with these presynaptic proteins that
also allow the synaptic vesicles to cluster at the active zones.
Termination of action of neurotransmitters
• The synaptic effects of a neurotransmitter are terminated by 3
mechanisms :
1. Transporter mediated uptake by presynaptic terminals or
astrocytes.
2. Enzymatic metabolism
3. Diffusion out of the synaptic cleft.
Termination of action of neurotransmitter
• Transporter mediated uptake by presynaptic terminal or astrocytes is the
sole mechanism for rapid termination of glutamate, GABA, Glycine and the
initial mechanism of inactivation of catecholamines and serotonin.
• Uptake involves Na+/ATP dependent uptake transporters which are plasma
membrane transporters. These are
1. SLC6 (Solute carrier 6 gene family) or Neurotransmitter-sodium
transporter family or Na+/Cl- dependent transporter
a. Monoamine transporter : Serotonin (SERT), Norepinephrine (NET),
Dopamine (DAT).
b. GABA (GAT)
c. Glycine
2. High affinity glutamate transporter or SLC1 gene family.
Termination of action of neurotransmitter
• In the case of DA, NE and serotonin, reuptake is followed by enzymatic
degradation to inactive metabolites by action of monoamine oxidases and
catechol-O-methyltransferases.
• In the case of NE, reuptake into the cytoplasm of the presynaptic terminal
(uptake 1) subserved by the NET is primarily responsible for its termination.
After uptake, some NE is enzymatically degraded by the mitochondrial
enzyme MAO, whereas additional fraction is retained in a cytoplasm. NE
can also be removed through the action of a transporter on the
postsynaptic membrane and in glia (uptake 2; subserved by the
extraneuronal monoamine transporter EMT). NE transported into the
postsynaptic neuron is degraded by the enzyme COMT.
• Enzymatic degradation is the sole mechanism for termination of the action
of Ach (acetylcholinesterase), Histamine (methyltansferase) and
Neuropeptides (peptidases) in the synaptic cleft.
Receptors and receptor subtypes
• Receptors are postsynaptic transmembrane proteins which exhibit affinity
for individual chemical messenger. There are 2 general types of receptors :
1. Ligand gated ion channel receptor or Ionotropic receptor : ion channels
that open in response to the chemical transmitter and allow the rapid
influx of cations (Na+ and Ca++) or anions (Cl-). This results in fast
excitatory or inhibitory postsynaptic responses respectively. This rapid
point to point transfer of information is referred to as Classic
Neurotransmission.
2. G protein coupled receptors : mediate slower changes in neuronal
excitability through activation or inhibition of K+ or Ca++ channels either
directly or through transduction pathways involving second messengers.
These effects are called Neuromodulation.
Receptors and receptor subtypes
• Receptors can also be classified according to the type of native
chemical messenger to which they respond.
• Example : cholinergic receptors, adrenergic receptors, GABA
receptors, Glutamate receptors.
Comparison of Classical Neurotransmission
and Neuromodulation
Feature Classic Neurotransmission Neuromodulation
Function Rapid synaptic excitation Regulation of neuronal excitability
or inhibition & neurotransmitter release
Receptor Ion channel receptors G protein coupled
Mechanisms (ligand gated) receptors
Ionic opening of Na+ or Ca++ opening or closing of
Mechanisms channel (fast EPSP) or voltage gated K+ or Ca++
Cl- channels (fast IPSP) channels
Comparison of Classical Neurotransmission
and Neuromodulation
Classic Neurotransmission Neuromodulation
Cationic channel receptors (Na+, Ca++) Metabotopic
1. Nicotinic Ach glutamate (mGluR1-8)
2. Ionotropic glutamate (AMPA, NMDA, Muscarinic Ach
Kainate) Monoamines
3. Serotonin (5HT3) GABAb
4. ATP Neuropeptides
Anionic channel receptors (Cl-)
1. GABAa
2. Glycine
Specific Neurochemical Systems
• The different neurochemical systems vary in distribution,
mechanism of action, and function. Amino acids are the
most abundant neurotransmitters in the CNS and are useful
for fast neurotransmission. Monoamines are less abundant
and produced more prolonged effects that are important in
modifying neural responses to amino acid neurotransmitters.
Neuropeptides are the least abundant but they are potent
and produce responses that have a long latency and long
duration.
Excitatory Amino Acid Systems
• L-Glutamate is the primary neurotransmitter of all excitatory
neurons in the CNS. This includes all pyramidal neurons of
the cerebral cortex and neurons in the relay nuclei of all
sensory and motor pathways.
• It is the most abundant amino acid in the brain.
Biosynthesis and reuptake of L-glutamate
• L-glutamate is derived from alpha ketoglutarate, an
intermediate metabolites of the Krebs cycle in neurons, and
from glutamine, which is synthesized in astrocytes. Through
the action of vesicular glutamate transporter, L-glutamate is
stored in small clear vesicles and released by exocytosis. The
synaptic effects of L-glutamate are terminated by ATP
dependent reuptake by the astrocytes via high affinity
glutamate transporters.
L-Glutamate Receptor Mechanism
• Glutamate acts through 2 main families of receptors : ionotropic
receptors and metabotropic receptors. Ionotropic glutamate
receptors are cation channels that mediate fast excitatory
transmission. These include Alpha amino 3 hydroxy 5 methyl 4
isoxazole proprionate (AMPA) receptor, Kainate receptors, and N
methyl D aspartate (NMDA). Metabotropic glutamate receptors
are G protein-coupled receptors that are involved in the
modulation of excitatory and inhibitory synapses. An important
result of the activation of glutamate receptors is increased levels
of cytosolic Ca++.
Role of Glutamate in Synaptic Plasticity:
• In several areas of the CNS, the strength of the excitatory
connection between presynaptic and postsynaptic neurons
exhibits a high degree of use-dependent plasticity. Synaptic
transmission may be either enhanced or depressed over time,
ranging from milliseconds to days or weeks or longer. The long
term increase in the strength of an excitatory synapse is called
long term potentiation and the converse phenomenon is called
long term depression. Both are critical for the establishment and
refinement of connections during brain development, for
memory functions, and for adaptive changes after injury of the
nervous system.
Role of Glutamate in Synaptic Plasticity:
• The mechanism of long term potentiation and long term
depression vary for different excitatory synapses and may
occur at both presynaptic and postsynaptic levels. In most
synapses, it involves the activation of glutamate receptors,
increase in intracellular Ca++, and activation of protein
kinases or phosphatases that affect the state of
phosphorylation of AMPA and NMDA receptors, other
synaptic proteins, or transcription factors, particularly CREB.
Inhibitory Amino Acid Systems
• The 2 main inhibitory amino acid neurotransmitters are
GABA and Glycine.
• GABA occurs in local inhibitory neurons of the cerebral
cortex, thalamus, and all sensory and motor relay nuclei.
GABA is also the primary neurotransmitter in circuits of the
basal ganglia and cerebellum involved in motor control.
Glycine mediates inhibitory transmission in the brainstem
and spinal cord.
Biosynthesis, reuptake and metabolism of
GABA
• The synthesis and metabolism of GABA are intimately linked
with those of L-glutamate and involve interactions between
GABAergic neurons and astrocytes. In GABAergic terminals,
GABA is synthesized from L-glutamate by the action of
glutamic acid decarboxylase. This enzyme requires pyridoxal
phosphate, a derivative of Vitamin B6 (pyridoxine). GABA is
incorporated into the synaptic vesicles by a vesicular GABA
transporter and released by exocytosis.
Biosynthesis, reuptake and metabolism of
GABA
• After release, GABA is taken up by astrocytes and
presynaptic GABAergic terminals (GAT). Following reuptake,
GABA is metabolized by GABA transaminase to an
intermediate that is metabolized through the Krebs cycle to
reconstitute alpha ketoglutarate (the precursor of
glutamate). In GABAergic neurons, glutamate is the source
of GABA. In contrast, astrocytes lack glutamic acid
decarboxylase and thus cannot reconstitute GABA.
Astrocytes contain glutamine synthetase and use glutamate
as a substrate for the biosynthesis of glutamine. This is a
critical mechanism for ammonia detoxification in the CNS.
GABA Receptor Mechanism
• The inhibitory actions of GABA are mediated by 2 classes of
receptors: GABAa receptors which are ligand gated Cl- channels,
and GABAb receptors which are G protein coupled receptors.
Activation of GABAa receptors trigger rapid influx of Cl- which in
most neurons produces hyperpolarization eliciting a fast
inhibition. Benzodiazepines, barbiturates and alcohol act on
this GABAa receptors. Activation of postsynaptic GABAb
receptors increases the permeability of voltage gated K+
channels which results in slow hyperpolarization and synaptic
inhibition. Activation of presynaptic GABAb receptors inhibits
the release of neurotransmitters.
Role of GABAergic neurons in the CNS
1. It acts as inhibitory interneuron in feed forward and feedback
circuits in all motor and sensory pathways. This basic circuit
consists of an excitatory axon that synapses on both an
excitatory projection (relay) neuron and a local GABAergic
neuron. In response to the excitatory afferent input, there is
monosynaptic excitation of the projection neuron and
disynaptic GABAergic inhibition of the same neuron, which
restricts the duration of activation.
2. Local GABAergic neurons inhibit other projection neurons that
surround the active neurons. This is called lateral inhibition.
This is an important mechanism for sensory discrimination and
fine motor control.
Role of GABAergic neurons in the CNS
3. In the cerebral cortex and hippocampus, GABAergic neurons prevent
the propagation of recurrent excitatory influences among pyramidal
neurons. Impairment of this inhibitory activity may result in paroxysmal
synchronized discharge of population of cortical pyramidal neurons and
cause a seizure.
4. Some GABAergic neurons from interconnected networks in the
cerebral cortex, thalamus and brainstem are important for the
synchronization of activity across widely distributed but functionally
related populations of neurons. During sleep, GABA is also the primary
neurotransmitter of neurons in the striatum and basal ganglia and of
Purkinje cells in the cerebellum. All these neurons are critical in motor
control circuits.
Inhibitory Amino Acid Systems
• Glycine in brainstem and spinal cord, contributes to fast
inhibitory postsynaptic transmission by acting on specific
glycine receptors. Like GABAa receptor, it allows rapid influx
of Cl-. These inhibitory glycine receptors are blocked by
strychnine, a toxin that produces severe hyperexcitability.
Cholinergic Systems
• Acetylcholine (Ach) is an important neurotransmitter in both the
central and peripheral nervous systems. Cholinergic systems include
1. spinal and brainstem somatic motor neurons innervating skeletal
muscles.
2. spinal and brainstem preganglionic neurons innervating autonomic
ganglia.
3. parasympathetic ganglion neurons innervating the viscera.
4. neurons in the basal forebrain (septal area and nucleus basali)
innervating the cerebral cortex.
5. neurons in the tegmemtum of the pons and midbrain innervating
the thalamus and medulla.
6. local neurons in the striatum.
Biosynthesis and metabolism of Ach
• Ach is synthesized from acetyl-coenzyme A and choline by
the action of choline acetyltransferase. Ach is incorporated
into synaptic vesicles by a vesicular Ach transporter and
released by exocytosis. The synaptic actions of Ach are
rapidly terminated through hydrolysis by
acetylcholinesterase. Drugs that inhibit this enzyme
(anticholinesterase agent) markedly potentiate cholinergic
transmission.
Ach Receptor Mechanisms
• Ach acts through 2 classes of receptors : nicotinic and
muscarinic. Nicotinic Ach receptors are ligand gated cation
channel receptors that allow the influx of Na or Ca++ (or
both) producing fast excitatory postsynaptic potentials in the
target cells. Muscarinic Ach receptors are G protein-coupled
receptors that mediate the slow excitatory (M1 type
receptors) or inhibitory (M2 type receptors) synaptic effects
of Ach.
Functions of Ach
1. It is the neurotransmitter at the neuromuscular junction that
acts through muscle type nicotinic receptors to elicit muscle
depolarization that leads to muscle contraction.
2. Preganglionic neurons in the brainstem and spinal cord release
Ach in the autonomic ganglia where Ach acts on ganglion-type
nicotinic receptors to activate sympathetic and parasympathetic
postganglionic neurons. Ach is the neurotransmitter of
parasympathetic ganglia neurons innervating all visceral organs
and sympathetic ganglia neurons innervating the sweat glands. It
acts on different subtypes of muscarinic receptors to control the
function of exocrine glands, visceral smooth muscle and the heart.
Functions of Ach
3. In the CNS, Ach has a major role in the mechanism of arousal,
attention, and memory. It is a critical neurotransmitter of neurons of
the consciousness system. Most of this central effects are mediated by
muscarinic receptors. However, the activation of presynaptic nicotinic
receptors regulates the release of many neurotransmitters.
4. Ach in the tegmentum of the pons and midbrain project to the
thalamus and other regions of the brainstem and are critical for arousal
and regulation of sleep-wake cycle.
5. Ach to the cerebral cortex arises from neurons in the basal forebrain
is important for attention and memory. These effects are mediated by
muscarinic M1 receptors which increase responses of cortical neurons
to excitatory inputs containing glutamate, thus facilitating long term
potentiation.
Dopaminergic Systems
• Dopamine is the neurotransmitter of 2 main groups of
neurons in the CNS : mesencephalic group and the
hypothalamic group.
• The mesencephalic group includes the substantia nigra pars
compacta and the ventral tegmental area. The substantia
nigra pars compacta innervates the striatum, and the ventral
tegmental area innervates the frontal lobes and limbic
system.
• The hypothalamic group controls the function of the anterior
pituitary.
Dopamine
• There are 5 Dopamine receptors
• D1 : brain, retina, kidney, and CV system.
• D2 : brain
• D3 : brain
• D4 : brain, retina
• D5 : brain
Dopamine Receptor Mechanism
• Dopamine receptors are G protein coupled receptors. It can
be subdivided into 2 main subfamilies : D1 and D2.
• D1 type receptors activate adenylate cyclase and trigger
cAMP dependent phosphorylation of different types of ion
channels and other proteins.
• D2 type receptors inhibit adenylate cyclase, activate K+
channels, inhibit Ca++ channels, and mediate the
presynaptic and postsynaptic inhibitory effects of dopamine.
Biosynthesis, reuptake and metabolism of
Dopamine
• Dopamine is synthesized from the amino acid L-tyrosine by the
action of tyrosine hydroxylase resulting in the production of L-
dopa (dihydroxyphenylalanine). L-dopa is metabolized by
aromatic amino acid decarboxylase to dopamine. Dopamine is
incorporated into synaptic vesicles by a vesicular monoamine
transporter coupled to the proton ATPase and released by
exocytosis. The synaptic effects are terminated by its reuptake by
a dopamine transporter located in the presynaptic dopaminergic
terminal. Once inside the terminal, dopamine is metabolized by
COMT and MAO to its final metabolite, homovanillic acid.
Functions of dopamine
1. Dopamine is important for motor control. Dopaminergic inputs from
the substantia nigra pars compacta and ventral tegmentum area to the
striatum provide a reward signal to the basal ganglia that initiate a
specific motor act at the expense of all other motor acts.
2. Its input from ventral tegmental area to the frontal lobe is important
for attention.
3. It is also important for endocrine function. The hypothalamic
dopamine influence on the anterior pituitary inhibits the secretion of
prolactin.
4. Dopaminergic neurons in the medulla are involved in the mechanism
of vomiting.
Noradrenergic, Serotonergic and
Histaminergic Systems
• Norepinephrine, serotonin, and histamine are the
neurotransmitters of the diffuse projection systems in
the brain. Diffuse projection system consists of
neurons located in the restricted regions of the
brainstem or hypothalamus whose axons provide
collaterals to widespread regions of the CNS.
Serotonin (5HT)
• 5HT is found in high concentrations in enterochromaffin cells
throughout the GI tract, platelets, and broadly throughout the CNS.
• There are 14 5-HT receptors. All of these receptors are G-protein
coupled receptors except 5-HT3 which is ligand gated receptor.
Noradrenergic, Serotonergic and
Histaminergic Systems
• The main source of noradrenergic innervations in the CNS is the
locus ceruleus located in the dorsal portion of the pons. Neurons
in this nucleus project to the cerebral cortex, basal ganglia,
thalamus, cerebellum, and sensory and motor nuclei. The lateral
tegmental system consists of neurons containing norepinephrine
or epinephrine that are located mainly in the reticular formation
of the ventrolateral medulla and innervate the hypothalamus
and autonomic nuclei of the brainstem and spinal cord. In the
periphery, norepinephrine is the neurotransmitter of
sympathetic ganglia that innervate all effector organs except the
sweat gland.
Noradrenergic, Serotonergic and
Histaminergic Systems
• Serotonin (5 hydroxytryptamine HT) is the neurotransmitter
of neurons in the raphe nuclei located in the midline along
the brainstem. They send ascending and descending
projections diffusely throughout the CNS
• Histamine neurons are located in the tuberomammillary
nucleus of the hypothalamus and their axons innervate all
areas of the CNS.
Biosynthesis, reuptake, and metabolism of
NE, E, and histamine
• Synthesis of NE is the same as dopamine. In noradrenergic neurons,
dopamine is transformed into NE by dopamine B-hydroxylase present
in the synaptic vesicle. In some neurons in the lateral tegmental area
and adrenal medulla, NE is transformed into epinephrine by
phenylalanine N-methyltransferase.
• Serotonin is synthesized from L-tryptophan by tryptophan
hydroxylase followed by decarboxylation by aromatic amino acid
decarboxylase.
• Histamine is synthesized from histidine by histidine decarboxylase.
Biosynthesis, reuptake, and metabolism of
NE, E, and histamine
• NE, serotonin and histamine are incorporated into synaptic vesicles by
a vesicular monoamine transporter. After release, they undergo
presynaptic reuptake by monoamine transporter except histamine.
• NE and serotonin are metabolized by monoamine oxidases and
histamine by methyltransferases. The metabolite of NE in the CNS is
3-methoxy 4-hydroxyphenylglycol, serotonin is 5-hydroxyindoleacetic
acid, and histamine is methylimidazoleacetic acid.
NE, E, and Histamine Receptor Mechanism
• The synaptic effects of NE, serotonin and histamine are complex and
mediated by different types of receptors.
• NE acts on alpha1, alpha2 and beta receptors including beta1 and beta2.
• Serotonin acts on 5HT1 to 5HT7.
• Histamine acts on H1, H2, H3.
• Except for 5HT3 receptors which are ligand gated ion channel, all
monoaminergic receptors are G protein coupled receptors that have
complex presynaptic and postsynaptic effects.
• alpha1, 5HT2, H1 receptors increase neuronal excitability.
• alpha2, 5HT1, H3 receptors elicit presynaptic and postsynaptic inhibition.
• Beta, 5HT4, H2 receptors activate adenyl cyclase and several cAMP
dependent phosphorylation cascades.
Functions of the Monoaminergic systems
• Through widespread projections and complex receptor
mechanisms, monoaminergic systems modulate the activity of
neuronal groups distributed throughout the brain and spinal
cord. They are involved in arousal, attention, and response to
stress including control of autonomic, and hypothalamic
functions, pain suppression and motor responses. They are
active during wakefulness and inactive during sleep.
• In the locus ceruleus, NE are activated in response to potentially
challenging environmental stimuli.
• In the periphery, NE is released from sympathetic terminals and
elicits effects like vasoconstriction (alpha1), stimulation of heart
(beta1), relaxation of visceral smooth muscle (Beta2).
Neuropeptide Systems
• Neuropeptides are abundant in the central and peripheral
nervous systems. In the CNS, the highest concentration is in the
hypothalamus followed by the amygdala, autonomic nuclei, and
the pain-modulating circuits of the brainstem and spinal cord.
Thus, they are important in the consciousness and internal
regulation systems. Examples of neuropeptides include
corticotrophin-releasing hormone (CRH), arginine vasopressin
(AVP), substance P, calcitonin gene-related peptide (CGRP),
vasoactive intestinal polypeptide (VIP), neuropeptide Y (NPY),
opioid peptides (including enkephalins, endorphins,
dysmorphins) and hypocretins (also called orexins).
Neuropeptide Systems
• Central peptidergic neurons are organized into 2 main
systems :
1. diffuse projection systems arising from the
hypothalamus, amygdala, and brainstem
2. local or short projection neurons located throughout
the CNS
• Neuropeptides frequently coexist with other
neurotransmitters including other neuropeptides, Ach,
monoamines or GABA.
Biosynthesis, release and processing of
neuropeptides
• Neuropeptides form several families. Members of each
family have common gene precursors, structural homologies
and functional similarities. Unlike other neurotransmitters,
neuropeptides are not synthesized in nerve terminals but in
the cell bodies from messenger RNA. They undergo
posttranslational modification within ER and Golgi apparatus
and are transported in large vesicles to the synaptic terminal
by fast anterograde axonal transport.
Biosynthesis, release and processing of
neuropeptides
• The release of neuropeptide is not restricted to presynaptic
active zones but occurs at voltage-gated Ca++ channels
distributed throughout the presynaptic terminal. In neurons
that contain both monoamines and neuropeptides,
continuous low frequency firing releases the monoamines
and high frequency burst firing releases the neuropeptides.
Neuropeptides do not undergo presynaptic reuptake. Their
action is terminated by hydrolysis by extracellular
peptidases.
Neuropeptide Receptor Mechanisms
• Neuropeptides act mainly as synaptic modulators and have potent
presynaptic and postsynaptic effects of slow onset and long duration.
These effects are mediated by G protein-coupled receptors.
• CRH, VIP and CGRP activate adenylcyclase.
• Substance P and hypocretins inhibit K+ channels and increase
neuronal excitability.
• Opioids activate K+ channels, reducing neuronal excitability,
and inhibit presynaptic Ca++ channels, reducing
neurotransmitter release.
• These prolonged modulatory effects occur not only at
postsynaptic sites but also in paracrine fashion by volume
transmission. Neuropeptides may act at a distance from the
site of release and thus affect neighboring neurons, glial cells
and blood vessels. Some hypothalamic neuropeptides are
released into the bloodstream (neuroendocrine effect).
Neuropeptides not only have neuromodulatory effect but
also trophic and vasomotor effects. Ex. VIP, CGRP, and
substance P produce vasodilatation. AVP and NPY cause
vasoconstriction.
Functions of Neuropeptides
• Neuropeptides exert a potent effect on endocrine, autonomic,
sensory, motor and behavioral functions in many cases by interacting
with other neurotransmitter systems. Many neuropeptides are critical
for specific homeostatic functions. Ex.
- CRH for stress
- AVP for fluid homeostasis
- Hypocretin for control of the sleep-cycle and food intake.
- Substance P and CGRP are neurotransmitters in nociceptive
afferents.
- NPY participates in sympathetic neurotransmission and VIP in
parasympathetic.
Other Neurochemical Messengers
• Purines : The purines, ATP and adenosine may act both as
neurotransmitters and neuromodulators in the central and
peripheral nervous systems.
• ATP acts through P2 – purinoreceptors and has important
functions in the nociceptive and autonomic systems. It is also
important in communication between astrocytes.
• Adenosine acts through P1 or adenosine receptors. It inhibits
the presynaptic release of other neurotransmitters and produces
vasodilatation.
Other Neurochemical Messengers
• Nitric Oxide is an important intercellular messenger
synthesized from arginine by nitric oxide synthase.
Constitutive forms of the enzyme are present in neurons and
endothelial cells and are activated by an increase in
intracellular Ca++ in response to activation of glutamate and
other neurotransmitter receptors. The inducible form is
present in macrophages and mononuclear cells and is
activated during inflammation.
Other Neurochemical Messengers
• Nitric oxide rapidly crosses membranes and reacts with the
iron contained in the heme molecule and in several key
enzymes including those in the mitochondrial respiratory
chain. Nitric oxide is an important synaptic modulator in the
central and autonomic nervous systems. They regulate
neuronal excitability and promote vasodilatation. Nitric oxide
is also said to be involved as a mediator of retrograde
transmission in the regulatory mechanism of presynaptic
terminal release of neurotransmission.
References
• 1. Mayo Clinic Medical Neurosciences by Bernaroch, Daube,
Flemming, and Westmoreland, 5th ed.
• 2. Fundamental Neurosciences for Basic and Clinical Applications by
Duane E. Haines, 4th ed.
• 3. Stahl’s Essential Psychopharmacology, Neuroscientific Basis and
Practical Application, 4th ed.
You might also like
- Video Recap of Cell Transport by Amoeba SistersDocument2 pagesVideo Recap of Cell Transport by Amoeba Sistersapi-2331875660% (4)
- Lecure-5 The Origin of Biopotentials - 2Document34 pagesLecure-5 The Origin of Biopotentials - 2Noor Ahmed100% (1)
- Biomedical Instrumentation I: Lecture-5: The Origin of BiopotentialsDocument35 pagesBiomedical Instrumentation I: Lecture-5: The Origin of BiopotentialsNitin PrajapatiNo ratings yet
- Introduction To RMP ND APDocument78 pagesIntroduction To RMP ND APHariharanNo ratings yet
- Nernst Equation: BY Dr.S.RajyalakshmiDocument12 pagesNernst Equation: BY Dr.S.RajyalakshmiRohit SreeramaNo ratings yet
- Basics On NeurophysiologyDocument12 pagesBasics On NeurophysiologyRausche Anne Blaser Sausa100% (1)
- Electrical Properties of BiomembranesDocument52 pagesElectrical Properties of Biomembranesc3rberussNo ratings yet
- Physiology Course: Dr. Velu M. RachelDocument18 pagesPhysiology Course: Dr. Velu M. RachelKunda JosephNo ratings yet
- Membrane Potentials Lecture 3Document54 pagesMembrane Potentials Lecture 3stehephNo ratings yet
- Lec. 11new - 05 - Membrane PotentialsDocument33 pagesLec. 11new - 05 - Membrane PotentialsDivine SundayNo ratings yet
- 2305 NeuronDocument5 pages2305 NeuronsridharNo ratings yet
- 2305 NeuronDocument5 pages2305 NeuronAnwar AsyhraffNo ratings yet
- 1.0 The Origin of Biopotentials (New)Document62 pages1.0 The Origin of Biopotentials (New)Hanif HussinNo ratings yet
- Membrane PotentialsDocument24 pagesMembrane PotentialsJeff ParkNo ratings yet
- Membrane Potential and Action Potential (1) - 1Document51 pagesMembrane Potential and Action Potential (1) - 1qvk8yy9pxcNo ratings yet
- Electro PhysiologyDocument59 pagesElectro PhysiologyjandaniellerasNo ratings yet
- Membrane PotentialsDocument40 pagesMembrane PotentialsdehaNo ratings yet
- 2.nerve PhysiologyDocument43 pages2.nerve Physiologycongresarad2022No ratings yet
- Membrane Potentials and Action PotentialsDocument24 pagesMembrane Potentials and Action PotentialsDenniel Dela Cuadra GasmenNo ratings yet
- Electrical Signals in NeuronDocument80 pagesElectrical Signals in NeuronmadwnNo ratings yet
- Membrane Potential: Dr. Arpana HazarikaDocument22 pagesMembrane Potential: Dr. Arpana HazarikaDorin PathakNo ratings yet
- Membrane Potentials.. Hassan ElalafDocument11 pagesMembrane Potentials.. Hassan Elalafislam rashadNo ratings yet
- Membrane Potential 2024Document29 pagesMembrane Potential 2024Zablon OtienoNo ratings yet
- NeurophysiologyDocument200 pagesNeurophysiologyashley nicholeNo ratings yet
- Resting Membrane PotentialDocument30 pagesResting Membrane PotentialMuhammad AhmedNo ratings yet
- Membrane PotentialDocument51 pagesMembrane PotentialDr. R. PeriasamyNo ratings yet
- NPB 100 Midterm 1 Study Guide-1Document4 pagesNPB 100 Midterm 1 Study Guide-1April MartínezNo ratings yet
- The Nerve IDocument17 pagesThe Nerve Inadine azmyNo ratings yet
- Bio621 Chapter 3Document109 pagesBio621 Chapter 3MizahNo ratings yet
- RestingMembranePotential RevisionDocument5 pagesRestingMembranePotential RevisionshaNo ratings yet
- Ent 311 - Medical Electronics and Bio-Instrumentation: WEEK: 4 & 5Document35 pagesEnt 311 - Medical Electronics and Bio-Instrumentation: WEEK: 4 & 5Nitin PrajapatiNo ratings yet
- Questions ExplanationDocument17 pagesQuestions ExplanationnomintmNo ratings yet
- NEURON ACTION POTENTIAL - PPT - ReadyDocument83 pagesNEURON ACTION POTENTIAL - PPT - Readyelle lee100% (1)
- Action PotentialDocument21 pagesAction PotentialjolilarmatarNo ratings yet
- 3 Excitable TissuesDocument32 pages3 Excitable TissuesSenthereng MoaisiNo ratings yet
- Action Potential in NeuronsDocument109 pagesAction Potential in NeuronsAdmin0% (1)
- Introduction To NeurophysiologyDocument13 pagesIntroduction To Neurophysiologyvenkata ramakrishnaiahNo ratings yet
- 15 - PDF - Basic Concepts of Excitable TissueDocument21 pages15 - PDF - Basic Concepts of Excitable Tissueshabnam sajidaNo ratings yet
- 03.resting Potential & Action PotentialDocument122 pages03.resting Potential & Action Potentialapi-19916399No ratings yet
- NB (019) An Introduction To Neurons and Communication Between Them UCL RevisedDocument38 pagesNB (019) An Introduction To Neurons and Communication Between Them UCL RevisedZeyad AmrNo ratings yet
- Biology 4822: Resting Membrane Potential Reviewed and ContinuedDocument30 pagesBiology 4822: Resting Membrane Potential Reviewed and ContinuedKimberly Parton BolinNo ratings yet
- Nervous System Topic 2 Lecture 4 5Document22 pagesNervous System Topic 2 Lecture 4 5amjad.46987No ratings yet
- Aim of This Class 2. A First Order Approximation of Neuronal BiophysicsDocument24 pagesAim of This Class 2. A First Order Approximation of Neuronal BiophysicsrajatNo ratings yet
- Membrane Physiology: Human Physiology By-Liul M. ForDocument34 pagesMembrane Physiology: Human Physiology By-Liul M. ForKhant Si ThuNo ratings yet
- 7.7 - Membrane Potential - Chemistry LibreTextsDocument5 pages7.7 - Membrane Potential - Chemistry LibreTextswihiw92934No ratings yet
- Electrical Properties of Cell MembraneDocument32 pagesElectrical Properties of Cell Membranemihaela irofte75% (4)
- Ch12 2018Document83 pagesCh12 2018김승윤No ratings yet
- Nervous SystemDocument6 pagesNervous SystemarellanokristelleNo ratings yet
- Fundamental Review Exam 1Document30 pagesFundamental Review Exam 1Beatrice RossNo ratings yet
- Action PotentialDocument24 pagesAction PotentialzzzNo ratings yet
- PL3102 Finals Revision NotesDocument77 pagesPL3102 Finals Revision NotesKatriel HeartsBigbangNo ratings yet
- Excitable Tissues AL 2021 Nerve HandoutDocument37 pagesExcitable Tissues AL 2021 Nerve Handoutpramodyasithumini202No ratings yet
- Encyc Human Brain 00Document31 pagesEncyc Human Brain 00humsuplohNo ratings yet
- Physiology Bio2424 1Document10 pagesPhysiology Bio2424 1Gopinath Govindaraj100% (1)
- Basic Concepts 8 BioelectricityDocument30 pagesBasic Concepts 8 BioelectricitydevdsantoshNo ratings yet
- Unit1 BERS Notes-Part2Document11 pagesUnit1 BERS Notes-Part2Mr. PavanNo ratings yet
- Electrical Potentials of NervesDocument6 pagesElectrical Potentials of Nervesابراهيم ماهر سلوم حسينNo ratings yet
- Physics ReportDocument6 pagesPhysics Reportابراهيم ماهر سلوم حسينNo ratings yet
- Electricity Within The Body Part 2Document31 pagesElectricity Within The Body Part 2ahmed husseinNo ratings yet
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- Experiment 3 - Frog NerveDocument29 pagesExperiment 3 - Frog NerveRachel CajilesNo ratings yet
- ch01 Ed PDFDocument18 pagesch01 Ed PDFRachel CajilesNo ratings yet
- EX 5 Rekative DensityDocument5 pagesEX 5 Rekative DensityRachel CajilesNo ratings yet
- Wilcoxon Signed-Rank Test: 4-Bio9, Group 10 Casipit, Legarda, YapDocument14 pagesWilcoxon Signed-Rank Test: 4-Bio9, Group 10 Casipit, Legarda, YapRachel CajilesNo ratings yet
- Formal Report Exp6Document7 pagesFormal Report Exp6Rachel CajilesNo ratings yet
- Electrophysiology & Electrotherapy For Clinical Practice Physiotherapy (Autosaved)Document60 pagesElectrophysiology & Electrotherapy For Clinical Practice Physiotherapy (Autosaved)Novita Dwi RachmawatiNo ratings yet
- Study Notes For Biology Exam 2Document19 pagesStudy Notes For Biology Exam 2YaffaAliNo ratings yet
- FA13 Cell Biology FinalDocument113 pagesFA13 Cell Biology FinalSana Savana Aman R100% (2)
- Ion ChannelsDocument35 pagesIon ChannelsnancyNo ratings yet
- Neurobiophysics2009 PreprintDocument52 pagesNeurobiophysics2009 PreprintsmiglemNo ratings yet
- Ligand Gated Ion ChannelDocument12 pagesLigand Gated Ion ChannelShoaib PatelNo ratings yet
- Lecture 2 PDFDocument15 pagesLecture 2 PDFAya MuhannadNo ratings yet
- Principles and Models of Biological Transport 2nd Ed - M. Friedman (Springer - 2008) WW PDFDocument520 pagesPrinciples and Models of Biological Transport 2nd Ed - M. Friedman (Springer - 2008) WW PDFAlexander FrischNo ratings yet
- Assignment of Pharmacology Submitted by Laiba Shah To DR MaryumDocument5 pagesAssignment of Pharmacology Submitted by Laiba Shah To DR MaryumLaiba ShahNo ratings yet
- Part 1 Test 2 2016 Name/Last Name - Date - Level A 1 Read The Text and Answer The Questions Choosing The Right AnswerDocument6 pagesPart 1 Test 2 2016 Name/Last Name - Date - Level A 1 Read The Text and Answer The Questions Choosing The Right AnswerAnnNo ratings yet
- Lecture On Resting Membrane Potential by DR RoomiDocument18 pagesLecture On Resting Membrane Potential by DR RoomiMudassar Roomi67% (3)
- Nerve FibresDocument35 pagesNerve Fibrestaniamahapatra1No ratings yet
- RegenerativeMedicineTissueEngineeringITO13 PDFDocument865 pagesRegenerativeMedicineTissueEngineeringITO13 PDFHeejin ChoiNo ratings yet
- For Presentation PapersDocument37 pagesFor Presentation PapersRachelle Zaragosa SolimanNo ratings yet
- Membrane and Subcellular BiochemistryDocument35 pagesMembrane and Subcellular BiochemistrylillyyynayyyNo ratings yet
- 8.1 The Nervous System and Nerve ImpulsesDocument60 pages8.1 The Nervous System and Nerve ImpulsesAmber Michaels100% (1)
- Neuromuscular PhysiologyDocument44 pagesNeuromuscular PhysiologyAnkita NigamNo ratings yet
- 01-02 MS Ion Channels PDFDocument30 pages01-02 MS Ion Channels PDF16_dev5038No ratings yet
- 2018 Book CMOSCircuitsForBiologicalSensiDocument354 pages2018 Book CMOSCircuitsForBiologicalSensiHoracio Dorantes ReyesNo ratings yet
- Lesson 5. Cell Transport NOTESDocument76 pagesLesson 5. Cell Transport NOTESPaolo RapanizaNo ratings yet
- Introduction To NeurophysiologyDocument13 pagesIntroduction To Neurophysiologyvenkata ramakrishnaiahNo ratings yet
- Membranes and Receptors PDFDocument242 pagesMembranes and Receptors PDFكرار عبدالحسين قاسمNo ratings yet
- Local Anesthesia - DR - Ibrahim - ShaikhDocument101 pagesLocal Anesthesia - DR - Ibrahim - ShaikhDrIbrahimShaikhNo ratings yet
- A Level Extended Response QuestionsDocument206 pagesA Level Extended Response QuestionsakilNo ratings yet
- Bertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewDocument18 pagesBertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewAmadorRevillaNo ratings yet
- UNESCO/IBRO Symposium: "Non-Conducting Membrane Mechanisms of Under-Threshold Signal Transduction in Neurons"Document5 pagesUNESCO/IBRO Symposium: "Non-Conducting Membrane Mechanisms of Under-Threshold Signal Transduction in Neurons"arevianNo ratings yet
- Pharmacology NotesDocument87 pagesPharmacology NotesAnonymous anXPzpy4100% (1)
- Cellular and Molecular NeurobiologyDocument24 pagesCellular and Molecular NeurobiologyDóra KristofóriNo ratings yet
- Biology 1 - 12 - Q1 - M8Document15 pagesBiology 1 - 12 - Q1 - M8Artlyne BunuanNo ratings yet