Professional Documents
Culture Documents
20.9A: The Nephelauxetic Effect: CR (III) - An Example in More Detail
20.9A: The Nephelauxetic Effect: CR (III) - An Example in More Detail
Uploaded by
Saurav PaulOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
20.9A: The Nephelauxetic Effect: CR (III) - An Example in More Detail
20.9A: The Nephelauxetic Effect: CR (III) - An Example in More Detail
Uploaded by
Saurav PaulCopyright:
Available Formats
20.
9A: The Nephelauxetic Effect
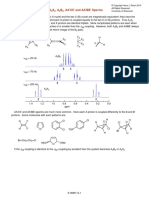
The spectra of the aqua ions for some first row transition metal ions are shown below.
For a much more detailed description of the interpretation of the spectra of first row transition metal ion complexes see
the notes on the use of Tanabe-Sugano diagrams.
Cr(III) - an example in more detail
For \9d^3\), d octahedral and
8
d
2
, d
7
tetrahedral complexes, the above diagrams can be used to interpret the observed
electronic absorption spectra.
Figure 2
Take for example the Cr aquo-ion [Cr(H O) ] . From the simplified Orgel diagram in Figure 2, three absorptions
3 +
2 6
3 +
transitions are expected. In practice, the spectrum is found to contain three bands which occur at 17,000 cm-1, 24,000 cm-1
and 37,000 cm-1. Of which only two are shown in Figure 1.
μ1 corresponds exactly to Δ (Delta) and since the lowest band is found at 17,000 cm-1 then this enables us to measure Δ
directly from the spectrum.
The next band is found at 24,000 cm-1 and this can be equated to:
μ = 9/5Δ − x (20.9A.1)
2
where x is the configuration interaction between the T(F) state and the T(P) state of the same symmetry.
Since Δ is 17,000 and μ2 is observed at 24,000 then x must be 6,600 cm-1.
The last band is seen at 37,000 cm-1 and here
μ3 = 6/5Δ + 15B + x (20.9A.2)
where B is one of the RACAH parameters.
Solving Equation 20.9A.2 for B gives a value of B = 667 cm −1
.
For the free Cr3+ ion, B is ~1030 cm-1 so that in the complex this term is reduced by ~2/3 of the free ion value.
A large reduction in B indicates a strong Nephelauxetic Effect. The Nephelauxetic Series is given by:
− − − − − − − −
F > H O > urea > NH > en\~ C O > NCS > Cl CN > Br > S2 I (20.9A.3)
2 3 2 42
Ionic ligands such as F give a small reduction in B , while covalently bonded ligands such as I give a large reduction of
− −
B.
Contributors
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)
Robert J. Lancashire 4/18/2020 20.9A.1 https://chem.libretexts.org/link?34406
You might also like
- Control Valves Sizing & SelectionDocument76 pagesControl Valves Sizing & SelectionABVSAI100% (7)
- StriplineDocument18 pagesStriplineJamilNo ratings yet
- Physics 1 Honors Formula SheetDocument11 pagesPhysics 1 Honors Formula SheetCody JohnsonNo ratings yet
- Fluid Flow Manual PDFDocument312 pagesFluid Flow Manual PDFsezaitanyolu100% (2)
- CH 11 Problems 5th EditionDocument3 pagesCH 11 Problems 5th Editionnisannn0% (1)
- Observation of Cyclotron Antiresonance in The Topological Insulator Bi TeDocument4 pagesObservation of Cyclotron Antiresonance in The Topological Insulator Bi TeSafi Ullah KhanNo ratings yet
- Inorganic Chap#3 And#4 Hom Take ExamDocument22 pagesInorganic Chap#3 And#4 Hom Take Examwold100% (1)
- Ppendix Ontact Adsorption of Ions: Derivation of The Triple Layer ModelDocument24 pagesPpendix Ontact Adsorption of Ions: Derivation of The Triple Layer ModellatiefNo ratings yet
- Material 2022Document19 pagesMaterial 2022saraNo ratings yet
- PHYS32600 Nanophysics 2019Document5 pagesPHYS32600 Nanophysics 2019mattNo ratings yet
- XII - Physics - Preboard 2 - Set BDocument7 pagesXII - Physics - Preboard 2 - Set BsidharthNo ratings yet
- 3.091 Introduction To Solid State ChemistryDocument11 pages3.091 Introduction To Solid State ChemistryDrew JenkinsNo ratings yet
- ISV SM Ch30Document46 pagesISV SM Ch30손은결No ratings yet
- Answers To Assignment2-2009Document6 pagesAnswers To Assignment2-2009ElmIeyHakiemiEyNo ratings yet
- Density of States in Graphene: Periodic Boundary ConditionDocument7 pagesDensity of States in Graphene: Periodic Boundary ConditionGemechis D DegagaNo ratings yet
- 8.321 Quantum Theory-I Fall 2016: Final Exam Dec 19, 2016Document4 pages8.321 Quantum Theory-I Fall 2016: Final Exam Dec 19, 2016bahadoor22i5583No ratings yet
- ECE330 Fall 16 Lecture2 PDFDocument9 pagesECE330 Fall 16 Lecture2 PDFPhùng Đức AnhNo ratings yet
- CH 2Document16 pagesCH 2Kashif AmjadNo ratings yet
- Group D Problem Set No 3 EE PC 4111Document7 pagesGroup D Problem Set No 3 EE PC 4111Hya Shane Tadlas TejumeNo ratings yet
- SECTION A (15 Marks) Answer ALL Questions in This SectionDocument15 pagesSECTION A (15 Marks) Answer ALL Questions in This SectionFazliawati MahayuddinNo ratings yet
- Strip LineDocument18 pagesStrip LineJamilNo ratings yet
- Physical Chemistry - Analysis of Organic CompoundsDocument7 pagesPhysical Chemistry - Analysis of Organic CompoundsChris FitzpatrickNo ratings yet
- Etc - Ei-Ii Final Exam Paper - 2020 - 21Document3 pagesEtc - Ei-Ii Final Exam Paper - 2020 - 21ARYA RAJPUTNo ratings yet
- Problem SetNo 3 4CC EE4CDocument7 pagesProblem SetNo 3 4CC EE4CrotcdublinNo ratings yet
- BLOCK II: Reasons of EMI: Division For Electricity and Lightning Research Uppsala UniversityDocument34 pagesBLOCK II: Reasons of EMI: Division For Electricity and Lightning Research Uppsala UniversityArnulfo SuarezNo ratings yet
- Electronic Properties of Material QuestionsDocument6 pagesElectronic Properties of Material Questionsaryan mike minzNo ratings yet
- Physics Xii SQPDocument9 pagesPhysics Xii SQPAYUSHI MANDALNo ratings yet
- hammer_hiremath_I_3Document24 pageshammer_hiremath_I_3Mahdi Norooz OliaeiNo ratings yet
- f - But the line reactance ωL increasesDocument20 pagesf - But the line reactance ωL increasesseelan9No ratings yet
- CoaxDocument6 pagesCoaxMeghan ButlerNo ratings yet
- Tanabe-Sugano DiagramsDocument5 pagesTanabe-Sugano DiagramsMa'arif A. SyafiiNo ratings yet
- Physics: Electromagnetism Spring 2007 PROBLEM SET 7 SolutionsDocument7 pagesPhysics: Electromagnetism Spring 2007 PROBLEM SET 7 SolutionsByron AlvaradoNo ratings yet
- Inductor DesignDocument18 pagesInductor DesignRRaman RewariaNo ratings yet
- Structure of Atom - QuestionDocument6 pagesStructure of Atom - Questionprakash bishtNo ratings yet
- IES 2003 EE Conventional Paper01Document5 pagesIES 2003 EE Conventional Paper01Shubham KumarNo ratings yet
- 2do Taller de Química Inorgánica IIDocument3 pages2do Taller de Química Inorgánica IIKaritto EspitiaNo ratings yet
- April 8th Shift 2 - Paper Solution (Combined)Document103 pagesApril 8th Shift 2 - Paper Solution (Combined)SHAKTI SWARUP SAHOONo ratings yet
- Annex 3 - Delivery Format - Task 3 Miguel PamplonaDocument17 pagesAnnex 3 - Delivery Format - Task 3 Miguel Pamplonamiguelitux martinezNo ratings yet
- Rollno. Anna University (University Departments) B.E. (Full Time) - End Semester Examinations, Nov/Dec 2021Document3 pagesRollno. Anna University (University Departments) B.E. (Full Time) - End Semester Examinations, Nov/Dec 2021hihelloNo ratings yet
- Z. L. Guo Et Al - Möbius Graphene Strip As A Topological InsulatorDocument8 pagesZ. L. Guo Et Al - Möbius Graphene Strip As A Topological InsulatorYlpkasoNo ratings yet
- Atomic Structure - Practice SheetDocument4 pagesAtomic Structure - Practice Sheetsameeryad72No ratings yet
- ASSIGNMENT I - 2023-24 - SEM II at UNIT I IIDocument3 pagesASSIGNMENT I - 2023-24 - SEM II at UNIT I IImagicianofgames518No ratings yet
- Exploring Competing Density Order in The Ionic Hubbard Model With Ultracold FermionsDocument8 pagesExploring Competing Density Order in The Ionic Hubbard Model With Ultracold FermionsAmy GillNo ratings yet
- Assignment 2 - Pool of Questions - SolutionDocument11 pagesAssignment 2 - Pool of Questions - SolutionNishant KhandelwalNo ratings yet
- Phy EndsemDocument2 pagesPhy EndsemK04Anoushka TripathiNo ratings yet
- 8863 Chapter 2Document8 pages8863 Chapter 2enjeeNo ratings yet
- Spectral Calculation of Orgel and Tanabe-Sugano Diagram'SDocument18 pagesSpectral Calculation of Orgel and Tanabe-Sugano Diagram'STiwari VishalNo ratings yet
- Orgel Diagram: Prof. Robert J. LancashireDocument5 pagesOrgel Diagram: Prof. Robert J. LancashireMa'arif A. Syafii0% (1)
- 10 Atomic StructureDocument9 pages10 Atomic StructurearcNo ratings yet
- Assignment Spectoscopy 1st Sem 2022Document7 pagesAssignment Spectoscopy 1st Sem 2022Debasish SharmaNo ratings yet
- Semiconductor Physics Problems 2015: A B C D E FDocument15 pagesSemiconductor Physics Problems 2015: A B C D E Fsan san moeNo ratings yet
- X-Ray Tutorial Solutions PDFDocument4 pagesX-Ray Tutorial Solutions PDFArjun MaharajNo ratings yet
- Massachusetts Institute of Technology: Reading AssignmentDocument3 pagesMassachusetts Institute of Technology: Reading AssignmentDibyananda SahooNo ratings yet
- A Comparative Study of The Bonding of N and CO To Ru (001) and The Role of 5 Orbital in Their Molecular Vibrational Frequency ChangesDocument3 pagesA Comparative Study of The Bonding of N and CO To Ru (001) and The Role of 5 Orbital in Their Molecular Vibrational Frequency ChangesTrần Duy TânNo ratings yet
- Physics Sample Papers 2022-23 KeyDocument28 pagesPhysics Sample Papers 2022-23 KeyOJASisLiveNo ratings yet
- TN SuganoDocument7 pagesTN SuganoYana SyafriyanaNo ratings yet
- Solution Set 7Document15 pagesSolution Set 7Jean AraúzNo ratings yet
- Kichael Carley Acoustics-24-30Document7 pagesKichael Carley Acoustics-24-30eki7777No ratings yet
- Neue Post', Apr 2011Document7 pagesNeue Post', Apr 2011emediageNo ratings yet
- Chp2-Planar Transmission LineswithexamplesDocument56 pagesChp2-Planar Transmission LineswithexamplesdhruvaaaaaNo ratings yet
- X-ray Absorption Spectroscopy for the Chemical and Materials SciencesFrom EverandX-ray Absorption Spectroscopy for the Chemical and Materials SciencesNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Voltagedependent Optical Activity of A Twisted Nematic Liquid CrystalDocument3 pagesVoltagedependent Optical Activity of A Twisted Nematic Liquid CrystalSaurav PaulNo ratings yet
- Funahashi 1997Document7 pagesFunahashi 1997Saurav PaulNo ratings yet
- Coates 1973Document2 pagesCoates 1973Saurav PaulNo ratings yet
- A Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionDocument2 pagesA Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionSaurav PaulNo ratings yet
- Dielectric Materials: Dielectric Materials Are and Used Principally in andDocument7 pagesDielectric Materials: Dielectric Materials Are and Used Principally in andSaurav PaulNo ratings yet
- Nakanishi 2004Document9 pagesNakanishi 2004Saurav PaulNo ratings yet
- 1455787138CHE P12 M29 EtextDocument7 pages1455787138CHE P12 M29 EtextSaurav PaulNo ratings yet
- Account Statement From 1 Oct 2020 To 6 Oct 2020Document1 pageAccount Statement From 1 Oct 2020 To 6 Oct 2020Saurav PaulNo ratings yet
- Structure Problem Solving Using H and C NMR Spectral Data Tutorial SessionDocument12 pagesStructure Problem Solving Using H and C NMR Spectral Data Tutorial SessionSaurav PaulNo ratings yet
- Basic Concept of C NMR: Subject ChemistryDocument15 pagesBasic Concept of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- 1455787432CHE P12 M33 E-TextDocument12 pages1455787432CHE P12 M33 E-TextSaurav PaulNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- 1455786764CHE P12 M24 E-TextDocument10 pages1455786764CHE P12 M24 E-TextSaurav PaulNo ratings yet
- 1455877785CHE P12 M35 EtextDocument8 pages1455877785CHE P12 M35 EtextSaurav PaulNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Towards Complex Matter: Supramolecular Chemistry and Self-OrganizationDocument19 pagesTowards Complex Matter: Supramolecular Chemistry and Self-OrganizationSaurav PaulNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- Account Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceDocument2 pagesAccount Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceSaurav PaulNo ratings yet
- Chapter 6 Introduction To Thermodynamics PDFDocument17 pagesChapter 6 Introduction To Thermodynamics PDFSaurav PaulNo ratings yet
- The Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldDocument4 pagesThe Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldSaurav PaulNo ratings yet
- Novel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USADocument4 pagesNovel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USASaurav PaulNo ratings yet
- The Structure of The Atom: Randima Piyumalie GalhenageDocument5 pagesThe Structure of The Atom: Randima Piyumalie GalhenageSaurav PaulNo ratings yet
- 41 Topics in Organometallic ChemistryDocument11 pages41 Topics in Organometallic ChemistrySaurav PaulNo ratings yet
- Primer Trig PhysicsDocument13 pagesPrimer Trig PhysicsjayNo ratings yet
- The Frenkel-Kontorova Model Concepts, Methods, and ApplicationsDocument491 pagesThe Frenkel-Kontorova Model Concepts, Methods, and ApplicationspatriciaterdalNo ratings yet
- Bolts TheoryDocument12 pagesBolts TheorybovingNo ratings yet
- Kinetic - Potential Energy (Grade 9) - Free Printable Tests and Worksheets - HelpTeachingDocument2 pagesKinetic - Potential Energy (Grade 9) - Free Printable Tests and Worksheets - HelpTeachingMOHDFADZLY84No ratings yet
- Chemical Bonding and Molecular StructureDocument14 pagesChemical Bonding and Molecular Structurepatelkrupa798No ratings yet
- Design of A Supersonic NozzleDocument39 pagesDesign of A Supersonic NozzlePandel0% (1)
- In The Name of Allah The Most Gracious The Most Merciful: Sensitivity: InternalDocument82 pagesIn The Name of Allah The Most Gracious The Most Merciful: Sensitivity: InternalAmina MunirNo ratings yet
- Optimization and Scale-Up of A Fluid Bed Tangential Spray Rotogranulation ProcessDocument9 pagesOptimization and Scale-Up of A Fluid Bed Tangential Spray Rotogranulation Processlakshya11No ratings yet
- CE 579 Lecture 3 Stability-Energy MethodDocument30 pagesCE 579 Lecture 3 Stability-Energy MethodbsitlerNo ratings yet
- Answers To End-Of-Chapter Questions: A B A B A, BDocument2 pagesAnswers To End-Of-Chapter Questions: A B A B A, BsybejoboNo ratings yet
- Problem SetDocument2 pagesProblem SetPhan MiNo ratings yet
- Dpp-3 (Electrostatic Potential)Document8 pagesDpp-3 (Electrostatic Potential)AayushNo ratings yet
- Mix Design of Semi Dense Bituminous ConcreteDocument18 pagesMix Design of Semi Dense Bituminous ConcreteRahul GoyalNo ratings yet
- MasteringPhysics Answers SOLUTIONSDocument139 pagesMasteringPhysics Answers SOLUTIONSgdfeiu dionwdn0% (1)
- New Regulations For Geotech GermanyDocument10 pagesNew Regulations For Geotech GermanySâu HeoNo ratings yet
- Tarea 5 TermodinamicaDocument4 pagesTarea 5 TermodinamicaMario GonzalezNo ratings yet
- 02 06ChapGere PDFDocument16 pages02 06ChapGere PDFPepe TejasNo ratings yet
- Soil-Structure Interaction For Building Structures: A ReviewDocument7 pagesSoil-Structure Interaction For Building Structures: A ReviewRahul KumarNo ratings yet
- Nanostructured Adsorbents: Ralph T. YangDocument46 pagesNanostructured Adsorbents: Ralph T. YangDario Bejarano RojasNo ratings yet
- Revision Exercises From Lecture SlidesDocument45 pagesRevision Exercises From Lecture SlidesANo ratings yet
- 10 1002@esp 5044Document41 pages10 1002@esp 5044VICTOR ANDRE SALINAS HERRERANo ratings yet
- Slope Deflection Method - Draft PDFDocument35 pagesSlope Deflection Method - Draft PDFRhina Dhel CarpioNo ratings yet
- A Tidal Flow Model For The Gulf of Kachchh IndiaDocument17 pagesA Tidal Flow Model For The Gulf of Kachchh IndiaYogeesh JayaramuNo ratings yet
- Quadcopter PDFDocument6 pagesQuadcopter PDFOla Fonda100% (1)
- LRFD Axially Loaded Compression MembersDocument4 pagesLRFD Axially Loaded Compression Membersالكشكولي رضوانNo ratings yet
- Atomic Structure (AP MC)Document4 pagesAtomic Structure (AP MC)Habiba AbdeenNo ratings yet