Professional Documents
Culture Documents
Application For The Grant / Renewal of A License To Manufacture For Sale of Ayurvedic / Siddha or Unani Drugs
Application For The Grant / Renewal of A License To Manufacture For Sale of Ayurvedic / Siddha or Unani Drugs
Uploaded by
Raghu NandanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Application For The Grant / Renewal of A License To Manufacture For Sale of Ayurvedic / Siddha or Unani Drugs
Application For The Grant / Renewal of A License To Manufacture For Sale of Ayurvedic / Siddha or Unani Drugs
Uploaded by
Raghu NandanCopyright:
Available Formats
ANNEXURE-I
FORM 24-D
[See Rule 153]
Application for the grant / renewal of a license to manufacture for sale of Ayurvedic / Siddha
or Unani drugs
1. I / We ………………………………….. of …………………………….
Hereby apply for the grant / renewal of a license to manufacture Ayurvedic (including
Siddha) or Unani drugs on the premises situated at……………………………………………
2. Names of Drugs to be manufactured (with details)
3. Names, qualification and experience of technical staff employed for
manufacture and testing of Ayurvedic (including Siddha) or Unani
drugs……………………………………………………..
4. A fee of rupees……………………………….has been credited to the
Government under the head of account……………………………………and the relevant
Treasury Challan is enclosed herewith.
Date………………….. Signature…….…………….………………..
(Applicant)
Note – The application should be accompanied by a plan of the premises.
You might also like
- Medical Fitness & Unfit CertificateDocument2 pagesMedical Fitness & Unfit Certificateotxcsundar40% (10)
- Medical Leave CertificateDocument1 pageMedical Leave Certificateprethishv67% (3)
- S - R - O - F-3-5-2013-DDC-Alt - MedDocument5 pagesS - R - O - F-3-5-2013-DDC-Alt - MedTayyab TahirNo ratings yet
- Guidance On Medical Device Establishment Licensing MDELDocument42 pagesGuidance On Medical Device Establishment Licensing MDELIss Shipper100% (1)
- Medical Essential Certificate-ADocument2 pagesMedical Essential Certificate-AShahed HusaainNo ratings yet
- Application For The Grant/renewal of A Licence To Manufacture For Sale of Ayurvedic/Siddha or Unani DrugsDocument1 pageApplication For The Grant/renewal of A Licence To Manufacture For Sale of Ayurvedic/Siddha or Unani DrugsDhaneeshNo ratings yet
- Form 24DDocument1 pageForm 24DDrSurendra Chaudhary100% (1)
- AYUSHmanufacturinglicenceDocument8 pagesAYUSHmanufacturinglicencesweetyyuvaniaNo ratings yet
- Form 19-CDocument15 pagesForm 19-Cs.sabapathyNo ratings yet
- DC Form24eDocument1 pageDC Form24eharikanth ademNo ratings yet
- Application For Grant or Renewal of A Licence To Manufacture Cosmetics For Sale (Or For Distribution)Document1 pageApplication For Grant or Renewal of A Licence To Manufacture Cosmetics For Sale (Or For Distribution)KunalGopalAgiwaleNo ratings yet
- Form-31 (See Rule 139)Document1 pageForm-31 (See Rule 139)devNo ratings yet
- Form CDDocument1 pageForm CDNiranjan KrishnaNo ratings yet
- Medical 1Document5 pagesMedical 1sahidNo ratings yet
- Medical Essential Certificate A BDocument2 pagesMedical Essential Certificate A BFayeem AnsariNo ratings yet
- Form 24-cDocument1 pageForm 24-cPaytmcare CustomerNo ratings yet
- She Rep and First AiderDocument2 pagesShe Rep and First AiderCharly MNNo ratings yet
- FSC GazettDocument2 pagesFSC GazettForamNo ratings yet
- Batch Plant OperatorDocument1 pageBatch Plant OperatorAsakundwi MukhwaNo ratings yet
- Form 12: (See Rule 34) Application For Licence To Import Drugs For Purpose of Examination, Test or AnalysisDocument1 pageForm 12: (See Rule 34) Application For Licence To Import Drugs For Purpose of Examination, Test or Analysissumit_waghmareNo ratings yet
- Form19C FORM 19 - C (See Rule 59Document1 pageForm19C FORM 19 - C (See Rule 59G Jai Prakash NaiduNo ratings yet
- Form-Guidelines For Ayurvedic ManufacturersDocument20 pagesForm-Guidelines For Ayurvedic ManufacturersRudrani SarkarNo ratings yet
- AS&U Drug Loan Lienc New Form 24EDocument1 pageAS&U Drug Loan Lienc New Form 24EprapannraghavNo ratings yet
- Application Form Grant of A LicenseDocument1 pageApplication Form Grant of A LicenseShahabWassiNo ratings yet
- Form 43Document2 pagesForm 43Rameshwar PagarNo ratings yet
- Application Form 2 For Grant or Renewal of An Establishment License To Import Medical DevicesDocument2 pagesApplication Form 2 For Grant or Renewal of An Establishment License To Import Medical DevicesOmerNo ratings yet
- Cosmetics Manufacturing License procedure-KMPDocument39 pagesCosmetics Manufacturing License procedure-KMPRx Girish MalaviyaNo ratings yet
- Application Form For Electrical Wiremen Competency To Work (Exemption) Grant or Renewal Form "A"Document2 pagesApplication Form For Electrical Wiremen Competency To Work (Exemption) Grant or Renewal Form "A"RAHUL KumarNo ratings yet
- Form 27FDocument1 pageForm 27Fsrinithish2No ratings yet
- Form-2 (Enlistment of Importer)Document2 pagesForm-2 (Enlistment of Importer)Farhan aliNo ratings yet
- mc8 FormDocument6 pagesmc8 FormthembaNo ratings yet
- India Patent Form 13Document2 pagesIndia Patent Form 13adityakochharNo ratings yet
- (FORM 32: (See Rule 140)Document2 pages(FORM 32: (See Rule 140)RohithNo ratings yet
- Covering Letter Drug Product (Permission)Document1 pageCovering Letter Drug Product (Permission)qafentumhealthcareNo ratings yet
- Final Guidance - Doc - Form-28 - 31-10-2012 PDFDocument40 pagesFinal Guidance - Doc - Form-28 - 31-10-2012 PDFAdvaitaNo ratings yet
- Schedule I Information Required For Registration of A CosmeticDocument2 pagesSchedule I Information Required For Registration of A CosmeticSuneth GunathilakaNo ratings yet
- Bangladesh Labor Rules 2015 English Version 15-09-2015-Pages-262-266Document5 pagesBangladesh Labor Rules 2015 English Version 15-09-2015-Pages-262-266Tanvir OveeNo ratings yet
- Form 25Document2 pagesForm 25gkk82No ratings yet
- Essentiality CertificateDocument4 pagesEssentiality CertificateRajesh KumarNo ratings yet
- Medical Certificate For Leave - Gaz OfficerDocument1 pageMedical Certificate For Leave - Gaz OfficerJayakrishna ReddyNo ratings yet
- Form 44 9 (India) Medical DeviceDocument2 pagesForm 44 9 (India) Medical DeviceAtrauliNo ratings yet
- Shlok Tripathi Aphthous UlcersDocument4 pagesShlok Tripathi Aphthous UlcersbesttubergamersNo ratings yet
- Medical Leave Proforma 20200612132007 - 2 - 2Document4 pagesMedical Leave Proforma 20200612132007 - 2 - 2besttubergamersNo ratings yet
- Form - A & Form-F: Application For Issuance of Certificate of PracticeDocument7 pagesForm - A & Form-F: Application For Issuance of Certificate of PracticeUmesh AraligidadNo ratings yet
- Form No. 19Document3 pagesForm No. 19Suafa TradersNo ratings yet
- Veterinary Biological 2Document63 pagesVeterinary Biological 2Gourav BhardwajNo ratings yet
- Docu For Fci PDFDocument3 pagesDocu For Fci PDFPIYUSH CHANDAKNo ratings yet
- Cosmetic Licence New Checklist 2022Document11 pagesCosmetic Licence New Checklist 2022ARIFNo ratings yet
- (See Rule 4 (2), 5 (1), and 6 (2) )Document2 pages(See Rule 4 (2), 5 (1), and 6 (2) )Enam HaqNo ratings yet
- Change of PremisesDocument9 pagesChange of PremisesSachin SoniNo ratings yet
- Standard Bidding Document HD 29.10.2015 SBDDocument65 pagesStandard Bidding Document HD 29.10.2015 SBDKhurramAslamNo ratings yet
- GN 02 Annex 1 Declaration For Exemption From Gdpmds (18aug)Document2 pagesGN 02 Annex 1 Declaration For Exemption From Gdpmds (18aug)Mary YamNo ratings yet
- Fertilizer LicenseDocument3 pagesFertilizer LicenseBharath VarmaNo ratings yet
- Form 16Document1 pageForm 16Vijay KrishnaNo ratings yet
- Guidance Document: (Medical Device and Diagnostics Division)Document2 pagesGuidance Document: (Medical Device and Diagnostics Division)DINESH GUMMADINo ratings yet
- Rajiv Gandhi Institute of Petroleum Technology, Rae BareliDocument2 pagesRajiv Gandhi Institute of Petroleum Technology, Rae BareliNilesh Kumar JhaNo ratings yet
- MC FormDocument3 pagesMC FormSreeraj B PillaiNo ratings yet
- Subtotalstempanswer 2Document16 pagesSubtotalstempanswer 2Raghu NandanNo ratings yet
- SubtotalstempDocument16 pagesSubtotalstempRaghu NandanNo ratings yet
- 4 0 0 Fertilizer Not Used 4 0 1 Fertilizer Used 8 0 5 0 5 0 4 0 8 0 4 0 7 1 6 1 6 1 7 1 8 1 8 1 7 1 6 1Document2 pages4 0 0 Fertilizer Not Used 4 0 1 Fertilizer Used 8 0 5 0 5 0 4 0 8 0 4 0 7 1 6 1 6 1 7 1 8 1 8 1 7 1 6 1Raghu NandanNo ratings yet
- MOU 3-15k Amazon and Flipkart 2 2Document4 pagesMOU 3-15k Amazon and Flipkart 2 2Raghu NandanNo ratings yet
- Dokumen - Tips - Quantitative Methods Problems Explanation 11 20 2012Document13 pagesDokumen - Tips - Quantitative Methods Problems Explanation 11 20 2012Raghu NandanNo ratings yet
- Source BibliographyDocument2 pagesSource BibliographyRaghu NandanNo ratings yet
- Door No.16-11-477/45, Sri Krishna Nilayam Dilsukhnagar Hyderabad TG 500036 INDocument1 pageDoor No.16-11-477/45, Sri Krishna Nilayam Dilsukhnagar Hyderabad TG 500036 INRaghu NandanNo ratings yet
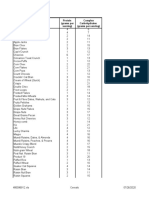
- Cereal Protein (Grams Per Serving) Complex Carbohydrates (Grams Per Serving)Document2 pagesCereal Protein (Grams Per Serving) Complex Carbohydrates (Grams Per Serving)Raghu NandanNo ratings yet
- Air Pollution An D Its Effects: M.Sunil Kumar M.N.V Raghu NandanDocument14 pagesAir Pollution An D Its Effects: M.Sunil Kumar M.N.V Raghu NandanRaghu NandanNo ratings yet
- Limit Allocation FormDocument1 pageLimit Allocation FormRaghu NandanNo ratings yet