Professional Documents
Culture Documents
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Uploaded by
Brayan Chaupis GrimaldoCopyright:
Available Formats
You might also like
- Chemical Equilibrium Multiple Choice QuestionsDocument4 pagesChemical Equilibrium Multiple Choice QuestionsCarol Mae Celis100% (7)
- Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)Document2 pagesMidterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)Juan Ramirez HuamanNo ratings yet
- ThermalMethodsAnalysis Haines - SolutionsDocument28 pagesThermalMethodsAnalysis Haines - SolutionsWalter Sperandio SampaioNo ratings yet
- 1983 - Quench Sensitivity of AlMgSi-Alloys Containing MN or CRDocument6 pages1983 - Quench Sensitivity of AlMgSi-Alloys Containing MN or CRzhaomingbaoNo ratings yet
- EllinghamDocument19 pagesEllinghamJuan Ignacio GonzálezNo ratings yet
- Use of Phase Diagrams in Studies of Refractories CorrosionDocument19 pagesUse of Phase Diagrams in Studies of Refractories CorrosionVictorNo ratings yet
- Ferdian 14060Document7 pagesFerdian 14060Marko JukićNo ratings yet
- Ferreira 2020Document6 pagesFerreira 2020ElnurNo ratings yet
- Sulfuric Acid Leaching of Sm-Co AlloyWaste and Separation of Samarium From CobaltDocument13 pagesSulfuric Acid Leaching of Sm-Co AlloyWaste and Separation of Samarium From CobaltWalter Sperandio SampaioNo ratings yet
- THM21-2 Main PDFDocument5 pagesTHM21-2 Main PDFONNDWELA RAMALAMULANo ratings yet
- Full Length Article: SciencedirectDocument9 pagesFull Length Article: Sciencedirectyh hvNo ratings yet
- CH 13Document30 pagesCH 13Laurertan TavaresNo ratings yet
- Thermal Expansion of SpinelsDocument6 pagesThermal Expansion of SpinelsRichardNo ratings yet
- A Quantitative Interpretation of DSC Experiments On Quenched and Aged Sicp Reinforced 8090 AlloysDocument6 pagesA Quantitative Interpretation of DSC Experiments On Quenched and Aged Sicp Reinforced 8090 AlloysRanjith KumarNo ratings yet
- Concepton Ellingham Diagram MetallurgyDocument18 pagesConcepton Ellingham Diagram MetallurgyArindam GoswamiNo ratings yet
- 4 - Chemical Bonding StructuredDocument28 pages4 - Chemical Bonding StructuredHayaa KhanNo ratings yet
- Description On Mass and Heat Balance ModDocument7 pagesDescription On Mass and Heat Balance ModGeorgi Mitkov SavovNo ratings yet
- 76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 CatalystDocument4 pages76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 Catalystrommy agurto palaciosNo ratings yet
- Aluminum Solubility in Bridgmanite Up To 3000 K at TH - 2021 - Geoscience FronDocument7 pagesAluminum Solubility in Bridgmanite Up To 3000 K at TH - 2021 - Geoscience FronchestherNo ratings yet
- Samarium Cobalt Magnets DatasheetDocument6 pagesSamarium Cobalt Magnets DatasheetOneil ZárateNo ratings yet
- IIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra PradeshDocument2 pagesIIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra PradeshshivaNo ratings yet
- Desulphurization by Slag TreatmentDocument22 pagesDesulphurization by Slag TreatmentanandvinaygeraNo ratings yet
- Slag/metal Reactions During Ladle Treatment With Focus On DesulphurisationDocument23 pagesSlag/metal Reactions During Ladle Treatment With Focus On DesulphurisationwefewfwefNo ratings yet
- Ellingham Diagram: Gibbs Free Energy Vs Temperature Diagrams For M-MO SystemsDocument25 pagesEllingham Diagram: Gibbs Free Energy Vs Temperature Diagrams For M-MO SystemsPransh KhubchandaniNo ratings yet
- Carbide Formation and Dissolution in Biomedical Co-Cr-Mo Alloys With Different Carbon Contents During Solution Treatment PDFDocument10 pagesCarbide Formation and Dissolution in Biomedical Co-Cr-Mo Alloys With Different Carbon Contents During Solution Treatment PDFJason AlexNo ratings yet
- Muh 1009 6Document8 pagesMuh 1009 6Chris BothaNo ratings yet
- BF 01161167Document46 pagesBF 01161167Andrei StefanNo ratings yet
- Dragrama de KelloggDocument6 pagesDragrama de KelloggIván Lautaro EspinozaNo ratings yet
- MgO Effect TGDocument3 pagesMgO Effect TGBiswanath senNo ratings yet
- A Flexible Dual Solid-Stateelectrolyte Supercapacitor With Suppressed Self-Discharge and Enhanced Stability SIDocument8 pagesA Flexible Dual Solid-Stateelectrolyte Supercapacitor With Suppressed Self-Discharge and Enhanced Stability SICB Dong SuwonNo ratings yet
- Supplementary Materials (Applied Catalysis B: Environmental)Document33 pagesSupplementary Materials (Applied Catalysis B: Environmental)anhchangcodon88No ratings yet
- Reduction of Chromium Oxide in Stainless Steel Slags: N. SanoDocument8 pagesReduction of Chromium Oxide in Stainless Steel Slags: N. SanoRam Deo AwasthiNo ratings yet
- Adv. Funct. Mater. 2016, 26, 7955-7964 Supp (MOH)Document21 pagesAdv. Funct. Mater. 2016, 26, 7955-7964 Supp (MOH)Chuah Chong YangNo ratings yet
- Effect of High-Temperature Heating On Chemical ChaDocument16 pagesEffect of High-Temperature Heating On Chemical ChaJarek PlaszczycaNo ratings yet
- No 137 Alfirano 3 MMTADocument10 pagesNo 137 Alfirano 3 MMTAJason LangNo ratings yet
- Precipitation of Metallic Chromium During Rapid Cooling of CR O SlagsDocument4 pagesPrecipitation of Metallic Chromium During Rapid Cooling of CR O SlagsChayon MondalNo ratings yet
- Formula Sheet Ctb3365Dwx - Drinking Water: Element Atomic Mass Element Atomic MassDocument2 pagesFormula Sheet Ctb3365Dwx - Drinking Water: Element Atomic Mass Element Atomic MassGiancarlo Raúl Manrique VillarrealNo ratings yet
- Smelting Technologies For FerrochromiumDocument14 pagesSmelting Technologies For FerrochromiumSantosh Kumar MahtoNo ratings yet
- Deposition and Characterization of Metalorganic ChemicalDocument7 pagesDeposition and Characterization of Metalorganic Chemicalunicum78No ratings yet
- Assignment 1 SteelmakingDocument2 pagesAssignment 1 SteelmakingVishal KumarNo ratings yet
- Homework06-Fig and TablesDocument4 pagesHomework06-Fig and TablesapiratzNo ratings yet
- Subpart 2: Physical Properties TablesDocument31 pagesSubpart 2: Physical Properties Tablesel_koptan00857693No ratings yet
- Considerations When Sintering Oxidation Sensitive PM SteelsDocument13 pagesConsiderations When Sintering Oxidation Sensitive PM SteelsSergey ZavadukNo ratings yet
- Phase Diagram of The Fe-C-V SystemDocument6 pagesPhase Diagram of The Fe-C-V SystemUlises Quintana CarhuanchoNo ratings yet
- SL - PAP - G.Zhang K.Chou - 2009 - Simple Method For Estimating The Electrical Conductivity of Oxide Melts WitDocument6 pagesSL - PAP - G.Zhang K.Chou - 2009 - Simple Method For Estimating The Electrical Conductivity of Oxide Melts WitEduardo CandelaNo ratings yet
- HCO Ca CO H: Calculation For SIDocument7 pagesHCO Ca CO H: Calculation For SIrinadonNo ratings yet
- EndSem MM454-Apr2014 Soln PDFDocument6 pagesEndSem MM454-Apr2014 Soln PDFPrakash ChandraNo ratings yet
- Materials: Liquid Regions of Lanthanum-Bearing AluminosilicatesDocument16 pagesMaterials: Liquid Regions of Lanthanum-Bearing Aluminosilicatesزھرة ٱلبيلسآنNo ratings yet
- Crystal Field Activation EnergyDocument5 pagesCrystal Field Activation EnergyBukhariNo ratings yet
- SMJK Chung Ling Pulau Pinang STPM Trial Exam Term 2 2019 (Chemistry)Document7 pagesSMJK Chung Ling Pulau Pinang STPM Trial Exam Term 2 2019 (Chemistry)AlyciaLeeNo ratings yet
- Corrosion Lecture ManchesterDocument88 pagesCorrosion Lecture ManchesterAli AbbasovNo ratings yet
- Determination of The Solubility Product and Enthalpy of Dissolution of Sparingly Soluble Salts by ConductometryDocument4 pagesDetermination of The Solubility Product and Enthalpy of Dissolution of Sparingly Soluble Salts by Conductometrykasun1237459No ratings yet
- TPR2Document10 pagesTPR2WILFREDO ROMAN PAUCARNo ratings yet
- Thermodynamic Data and E PH DiagramsDocument23 pagesThermodynamic Data and E PH DiagramsManuel CruzNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument17 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsWissal ZitouniNo ratings yet
- Alloying ElementsDocument6 pagesAlloying ElementskarthisekarNo ratings yet
- Lecture 8: Thermo Chemistry Applications in Metal ExtractionDocument6 pagesLecture 8: Thermo Chemistry Applications in Metal ExtractionYudha PradhanaNo ratings yet
- Recitation 2 QuestionsDocument14 pagesRecitation 2 QuestionsfzfwsbyxrhNo ratings yet
- P92 Paper IIW Conference GrazDocument13 pagesP92 Paper IIW Conference GrazzhangxingzydNo ratings yet
- 4516 - Stal S51-34Document1 page4516 - Stal S51-34Adrián EcheverríaNo ratings yet
- Chapter 3.0Document3 pagesChapter 3.0Jhon Barzola PalominoNo ratings yet
- Test 1 With AnsDocument4 pagesTest 1 With AnsKavinesh GanesanNo ratings yet
- WBGT - Waterless Wetbulb - For WebDocument4 pagesWBGT - Waterless Wetbulb - For WebandrewhwNo ratings yet
- Stirling Engine & ApplicationsDocument32 pagesStirling Engine & ApplicationsLASINNo ratings yet
- Assessment of Sustainability Indicators of Two Gas - Turbine Plants With Naphtha and Naphtha-Rfg Mixture As FuelsDocument14 pagesAssessment of Sustainability Indicators of Two Gas - Turbine Plants With Naphtha and Naphtha-Rfg Mixture As FuelsGlobal Research and Development ServicesNo ratings yet
- Intershipe Report Aditya HVACDocument54 pagesIntershipe Report Aditya HVACAmol GaikarNo ratings yet
- Chillers and Boilers Parts ListDocument29 pagesChillers and Boilers Parts ListAwais JalaliNo ratings yet
- Refrigeration Systems Fme19Document9 pagesRefrigeration Systems Fme19richmond alcalaNo ratings yet
- A.2 - The Standard Atmosphere PDFDocument5 pagesA.2 - The Standard Atmosphere PDFf7bio7assun77o-1No ratings yet
- Ficha Técnica Orion RKLDocument2 pagesFicha Técnica Orion RKLOscar MartinezNo ratings yet
- Assignment02 KM32203Document3 pagesAssignment02 KM32203Raiyre RolandNo ratings yet
- Mathematical Procedure For Predicting Tube Metal Temperature in The Second Stage Reheater of The Operating Flexibly Steam BoilerDocument12 pagesMathematical Procedure For Predicting Tube Metal Temperature in The Second Stage Reheater of The Operating Flexibly Steam BoilerraitoNo ratings yet
- Report HVAC Exp1 UthmDocument5 pagesReport HVAC Exp1 UthmIdinNo ratings yet
- MHM 420 - 780 2018 Final ExamDocument3 pagesMHM 420 - 780 2018 Final ExamNicholas RuestNo ratings yet
- Make Up Water Calculation For Cooling TowerDocument2 pagesMake Up Water Calculation For Cooling TowerSiLan Subramaniam100% (1)
- Lecture 7: Isothermal Flash CalculationsDocument19 pagesLecture 7: Isothermal Flash CalculationsAREEJ JAVEDNo ratings yet
- BODR For HVACDocument3 pagesBODR For HVACMo EmadNo ratings yet
- Chapter 12 - Heat ExchangerDocument21 pagesChapter 12 - Heat ExchangerBảo Tín TrầnNo ratings yet
- Thermochemistry IB QuestionsDocument4 pagesThermochemistry IB QuestionsArmstrong NworkaNo ratings yet
- Worksheet - Melting Point DeterminationDocument4 pagesWorksheet - Melting Point DeterminationSobi SitjarNo ratings yet
- Science 7 Heat Transfer Learning Activity Sheets 6Document3 pagesScience 7 Heat Transfer Learning Activity Sheets 6fitz zamoraNo ratings yet
- BME Unit 1Document40 pagesBME Unit 1Renu PeriketiNo ratings yet
- 3 - CET-II - Solution Thermodynamics-App PDFDocument48 pages3 - CET-II - Solution Thermodynamics-App PDFshilp patelNo ratings yet
- Vapour Compression RefrigerationDocument4 pagesVapour Compression RefrigerationAhmadNo ratings yet
- ASTM-E-145-94 (Reapproved 2006) Standard Specification For Gravity-Convection and Forced-Ventilation Ovens1Document3 pagesASTM-E-145-94 (Reapproved 2006) Standard Specification For Gravity-Convection and Forced-Ventilation Ovens1Ben Aguilar100% (1)
- PCM Heat SinkDocument7 pagesPCM Heat SinkSachin BelgaonkarNo ratings yet
- Mono Splits York InverterDocument4 pagesMono Splits York Inverterbride junior tchuensuNo ratings yet
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Uploaded by
Brayan Chaupis GrimaldoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)
Uploaded by
Brayan Chaupis GrimaldoCopyright:
Available Formats
Midterm Examination School of
UNI Metallurgical
FIGMM Fundamentals of Metalurgy I Engineering
1.- Conversion of copper matte (Cu2S-FeS)
With the help of the thermodynamic data determine if at 1300 ° C the metal phase is mainly formed by Cu (l) and the slag phase is mostly conformed by
fayalite. The slag phase can retain up to 15% Cu. Proposes a method of recovering this metal phase.
We ask:
a.- Write the reactions as to be3represented in an Ellingham diagram.
b.- Establish the reductive order4 for the chosen metals at a temperature of 1300 ° C.
c.- Calculate the DHº average and the DSº average of any of the reactions and indicate if it is exothermic
d.- For the studied temperature range, its increase favors the formation of slag and Cu (l).
Reaction G =A + B*T(kj/mol) Temperarature range (°C)
D (1,T)
A B from to
Cu2S(l) //2Cu(l) + SO2(g) -218.62898 0.03127 1080 1300
4Cu(l)// 2Cu2O(l) -252.09560 0.09240 1080 1300
2Cu2O(l) + Cu2S(l) //6Cu(l) 33.46662 -0.06113 1080 1300
0.25Cu2S(l)+0.5FeS(l) +0.25SiO2 //0.5Cu(l) + 0.25Fe2SiO4(F) + 0.75SO2(g) -314.92910 0.06200 1080 1300
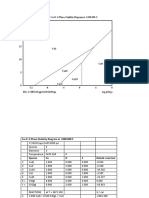
2.- DIAGRAM Cr-S-O
Based on the graph (referential diagram) and attachment data:
a) Write the reactions shown in the attached chart to define the stability zone of the Cr2O3.
b) Demonstrate the analytical form of the phase rule for the accompanying equation graph. Apply the phase rule and note the invariant, monovariant and bivariate
equilibria of the represented system.
c) With the aid of the free enthalpy of formation and of the entropy of the present species to 25°C calculate the Keq and define (coordenates) the area of Cr 2O3., Compare
with the area of the presented diagram and for any invariant point of the indicated area find the% error between the reference and the calculation made for any coordenate of the points
of the area.
d) If the roasting oven operates at1200 °C and at % SO2= 10 and % O2= 5; define the product of chrome roasting. Indicate whether it is a sulphating or toasting. This
same product of roasting is in the nature at 25°. Indicate a treatment method to recover the Cr form the product formed.
Diagram Cr-S-O at 1250 ˚C
log pSO2(g) Cr-O -S Phase Stability Diagram at 1250.000 C
20
15
10 Cr2(SO4)3 ° S°
Specie m i 25°C i 25°C
5
4 kjoule/mol joule/mol°K
Cr 0.000 23.543
Cr2S3
3 Cr2O3 -1053.111 81.154
0
-5 Cr2O3 -501.227 63.178
CrO3
CrS -137.799 65
-10 5 CrO3
2 -365.3 147.628
Cr2S3
CrS
1
-15 Cr2(SO4)3 -2578.25 258.78
Cr 1 SO2 (g)
-300.093 248.220
-20
(g)
-25 -20 -15 -10 -5 0 5 10 15 20 O2 0.000 205.149
File: C:\HSC6\Lpp\CrOS1250.ips log pO2(g)
Ciclo 2016-II OSC 12-X-16
3.- PHASE DIAGRAM
The Au-Sn binary diagram is attached.
a) Demonstrate the phase rule for the binary and apply it by pointing to bivariate, monovariant and invariant equilibria. Point out the components and constituents of
the system.
b) Indicate the solids to melt congruent and incongruent, if any, indicate their respective melting points.
c) Indicate the approximate temperature of occurrence of the chosen invariants and their respective reactions.
d) Is there any invariant solid state reaction?
e) Express in fraction by weight the molar fraction of the intermetallic compound d .at %w Sn. Atm W. Au= 196.97 g/mol, Atm. W. Sn =118.69 g/mol.
Ciclo 2016-II OSC 12-X-16
You might also like
- Chemical Equilibrium Multiple Choice QuestionsDocument4 pagesChemical Equilibrium Multiple Choice QuestionsCarol Mae Celis100% (7)
- Midterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)Document2 pagesMidterm Examination: 1.-Conversion of Copper Matte (Cu S-Fes)Juan Ramirez HuamanNo ratings yet
- ThermalMethodsAnalysis Haines - SolutionsDocument28 pagesThermalMethodsAnalysis Haines - SolutionsWalter Sperandio SampaioNo ratings yet
- 1983 - Quench Sensitivity of AlMgSi-Alloys Containing MN or CRDocument6 pages1983 - Quench Sensitivity of AlMgSi-Alloys Containing MN or CRzhaomingbaoNo ratings yet
- EllinghamDocument19 pagesEllinghamJuan Ignacio GonzálezNo ratings yet
- Use of Phase Diagrams in Studies of Refractories CorrosionDocument19 pagesUse of Phase Diagrams in Studies of Refractories CorrosionVictorNo ratings yet
- Ferdian 14060Document7 pagesFerdian 14060Marko JukićNo ratings yet
- Ferreira 2020Document6 pagesFerreira 2020ElnurNo ratings yet
- Sulfuric Acid Leaching of Sm-Co AlloyWaste and Separation of Samarium From CobaltDocument13 pagesSulfuric Acid Leaching of Sm-Co AlloyWaste and Separation of Samarium From CobaltWalter Sperandio SampaioNo ratings yet
- THM21-2 Main PDFDocument5 pagesTHM21-2 Main PDFONNDWELA RAMALAMULANo ratings yet
- Full Length Article: SciencedirectDocument9 pagesFull Length Article: Sciencedirectyh hvNo ratings yet
- CH 13Document30 pagesCH 13Laurertan TavaresNo ratings yet
- Thermal Expansion of SpinelsDocument6 pagesThermal Expansion of SpinelsRichardNo ratings yet
- A Quantitative Interpretation of DSC Experiments On Quenched and Aged Sicp Reinforced 8090 AlloysDocument6 pagesA Quantitative Interpretation of DSC Experiments On Quenched and Aged Sicp Reinforced 8090 AlloysRanjith KumarNo ratings yet
- Concepton Ellingham Diagram MetallurgyDocument18 pagesConcepton Ellingham Diagram MetallurgyArindam GoswamiNo ratings yet
- 4 - Chemical Bonding StructuredDocument28 pages4 - Chemical Bonding StructuredHayaa KhanNo ratings yet
- Description On Mass and Heat Balance ModDocument7 pagesDescription On Mass and Heat Balance ModGeorgi Mitkov SavovNo ratings yet
- 76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 CatalystDocument4 pages76 Direct Hydrogenation of Aliphatic Carboxylic Acids To Corresponding Aldehydes With Cr203 Catalystrommy agurto palaciosNo ratings yet
- Aluminum Solubility in Bridgmanite Up To 3000 K at TH - 2021 - Geoscience FronDocument7 pagesAluminum Solubility in Bridgmanite Up To 3000 K at TH - 2021 - Geoscience FronchestherNo ratings yet
- Samarium Cobalt Magnets DatasheetDocument6 pagesSamarium Cobalt Magnets DatasheetOneil ZárateNo ratings yet
- IIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra PradeshDocument2 pagesIIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra PradeshshivaNo ratings yet
- Desulphurization by Slag TreatmentDocument22 pagesDesulphurization by Slag TreatmentanandvinaygeraNo ratings yet
- Slag/metal Reactions During Ladle Treatment With Focus On DesulphurisationDocument23 pagesSlag/metal Reactions During Ladle Treatment With Focus On DesulphurisationwefewfwefNo ratings yet
- Ellingham Diagram: Gibbs Free Energy Vs Temperature Diagrams For M-MO SystemsDocument25 pagesEllingham Diagram: Gibbs Free Energy Vs Temperature Diagrams For M-MO SystemsPransh KhubchandaniNo ratings yet
- Carbide Formation and Dissolution in Biomedical Co-Cr-Mo Alloys With Different Carbon Contents During Solution Treatment PDFDocument10 pagesCarbide Formation and Dissolution in Biomedical Co-Cr-Mo Alloys With Different Carbon Contents During Solution Treatment PDFJason AlexNo ratings yet
- Muh 1009 6Document8 pagesMuh 1009 6Chris BothaNo ratings yet
- BF 01161167Document46 pagesBF 01161167Andrei StefanNo ratings yet
- Dragrama de KelloggDocument6 pagesDragrama de KelloggIván Lautaro EspinozaNo ratings yet
- MgO Effect TGDocument3 pagesMgO Effect TGBiswanath senNo ratings yet
- A Flexible Dual Solid-Stateelectrolyte Supercapacitor With Suppressed Self-Discharge and Enhanced Stability SIDocument8 pagesA Flexible Dual Solid-Stateelectrolyte Supercapacitor With Suppressed Self-Discharge and Enhanced Stability SICB Dong SuwonNo ratings yet
- Supplementary Materials (Applied Catalysis B: Environmental)Document33 pagesSupplementary Materials (Applied Catalysis B: Environmental)anhchangcodon88No ratings yet
- Reduction of Chromium Oxide in Stainless Steel Slags: N. SanoDocument8 pagesReduction of Chromium Oxide in Stainless Steel Slags: N. SanoRam Deo AwasthiNo ratings yet
- Adv. Funct. Mater. 2016, 26, 7955-7964 Supp (MOH)Document21 pagesAdv. Funct. Mater. 2016, 26, 7955-7964 Supp (MOH)Chuah Chong YangNo ratings yet
- Effect of High-Temperature Heating On Chemical ChaDocument16 pagesEffect of High-Temperature Heating On Chemical ChaJarek PlaszczycaNo ratings yet
- No 137 Alfirano 3 MMTADocument10 pagesNo 137 Alfirano 3 MMTAJason LangNo ratings yet
- Precipitation of Metallic Chromium During Rapid Cooling of CR O SlagsDocument4 pagesPrecipitation of Metallic Chromium During Rapid Cooling of CR O SlagsChayon MondalNo ratings yet
- Formula Sheet Ctb3365Dwx - Drinking Water: Element Atomic Mass Element Atomic MassDocument2 pagesFormula Sheet Ctb3365Dwx - Drinking Water: Element Atomic Mass Element Atomic MassGiancarlo Raúl Manrique VillarrealNo ratings yet
- Smelting Technologies For FerrochromiumDocument14 pagesSmelting Technologies For FerrochromiumSantosh Kumar MahtoNo ratings yet
- Deposition and Characterization of Metalorganic ChemicalDocument7 pagesDeposition and Characterization of Metalorganic Chemicalunicum78No ratings yet
- Assignment 1 SteelmakingDocument2 pagesAssignment 1 SteelmakingVishal KumarNo ratings yet
- Homework06-Fig and TablesDocument4 pagesHomework06-Fig and TablesapiratzNo ratings yet
- Subpart 2: Physical Properties TablesDocument31 pagesSubpart 2: Physical Properties Tablesel_koptan00857693No ratings yet
- Considerations When Sintering Oxidation Sensitive PM SteelsDocument13 pagesConsiderations When Sintering Oxidation Sensitive PM SteelsSergey ZavadukNo ratings yet
- Phase Diagram of The Fe-C-V SystemDocument6 pagesPhase Diagram of The Fe-C-V SystemUlises Quintana CarhuanchoNo ratings yet
- SL - PAP - G.Zhang K.Chou - 2009 - Simple Method For Estimating The Electrical Conductivity of Oxide Melts WitDocument6 pagesSL - PAP - G.Zhang K.Chou - 2009 - Simple Method For Estimating The Electrical Conductivity of Oxide Melts WitEduardo CandelaNo ratings yet
- HCO Ca CO H: Calculation For SIDocument7 pagesHCO Ca CO H: Calculation For SIrinadonNo ratings yet
- EndSem MM454-Apr2014 Soln PDFDocument6 pagesEndSem MM454-Apr2014 Soln PDFPrakash ChandraNo ratings yet
- Materials: Liquid Regions of Lanthanum-Bearing AluminosilicatesDocument16 pagesMaterials: Liquid Regions of Lanthanum-Bearing Aluminosilicatesزھرة ٱلبيلسآنNo ratings yet
- Crystal Field Activation EnergyDocument5 pagesCrystal Field Activation EnergyBukhariNo ratings yet
- SMJK Chung Ling Pulau Pinang STPM Trial Exam Term 2 2019 (Chemistry)Document7 pagesSMJK Chung Ling Pulau Pinang STPM Trial Exam Term 2 2019 (Chemistry)AlyciaLeeNo ratings yet
- Corrosion Lecture ManchesterDocument88 pagesCorrosion Lecture ManchesterAli AbbasovNo ratings yet
- Determination of The Solubility Product and Enthalpy of Dissolution of Sparingly Soluble Salts by ConductometryDocument4 pagesDetermination of The Solubility Product and Enthalpy of Dissolution of Sparingly Soluble Salts by Conductometrykasun1237459No ratings yet
- TPR2Document10 pagesTPR2WILFREDO ROMAN PAUCARNo ratings yet
- Thermodynamic Data and E PH DiagramsDocument23 pagesThermodynamic Data and E PH DiagramsManuel CruzNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument17 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsWissal ZitouniNo ratings yet
- Alloying ElementsDocument6 pagesAlloying ElementskarthisekarNo ratings yet
- Lecture 8: Thermo Chemistry Applications in Metal ExtractionDocument6 pagesLecture 8: Thermo Chemistry Applications in Metal ExtractionYudha PradhanaNo ratings yet
- Recitation 2 QuestionsDocument14 pagesRecitation 2 QuestionsfzfwsbyxrhNo ratings yet
- P92 Paper IIW Conference GrazDocument13 pagesP92 Paper IIW Conference GrazzhangxingzydNo ratings yet
- 4516 - Stal S51-34Document1 page4516 - Stal S51-34Adrián EcheverríaNo ratings yet
- Chapter 3.0Document3 pagesChapter 3.0Jhon Barzola PalominoNo ratings yet
- Test 1 With AnsDocument4 pagesTest 1 With AnsKavinesh GanesanNo ratings yet
- WBGT - Waterless Wetbulb - For WebDocument4 pagesWBGT - Waterless Wetbulb - For WebandrewhwNo ratings yet
- Stirling Engine & ApplicationsDocument32 pagesStirling Engine & ApplicationsLASINNo ratings yet
- Assessment of Sustainability Indicators of Two Gas - Turbine Plants With Naphtha and Naphtha-Rfg Mixture As FuelsDocument14 pagesAssessment of Sustainability Indicators of Two Gas - Turbine Plants With Naphtha and Naphtha-Rfg Mixture As FuelsGlobal Research and Development ServicesNo ratings yet
- Intershipe Report Aditya HVACDocument54 pagesIntershipe Report Aditya HVACAmol GaikarNo ratings yet
- Chillers and Boilers Parts ListDocument29 pagesChillers and Boilers Parts ListAwais JalaliNo ratings yet
- Refrigeration Systems Fme19Document9 pagesRefrigeration Systems Fme19richmond alcalaNo ratings yet
- A.2 - The Standard Atmosphere PDFDocument5 pagesA.2 - The Standard Atmosphere PDFf7bio7assun77o-1No ratings yet
- Ficha Técnica Orion RKLDocument2 pagesFicha Técnica Orion RKLOscar MartinezNo ratings yet
- Assignment02 KM32203Document3 pagesAssignment02 KM32203Raiyre RolandNo ratings yet
- Mathematical Procedure For Predicting Tube Metal Temperature in The Second Stage Reheater of The Operating Flexibly Steam BoilerDocument12 pagesMathematical Procedure For Predicting Tube Metal Temperature in The Second Stage Reheater of The Operating Flexibly Steam BoilerraitoNo ratings yet
- Report HVAC Exp1 UthmDocument5 pagesReport HVAC Exp1 UthmIdinNo ratings yet
- MHM 420 - 780 2018 Final ExamDocument3 pagesMHM 420 - 780 2018 Final ExamNicholas RuestNo ratings yet
- Make Up Water Calculation For Cooling TowerDocument2 pagesMake Up Water Calculation For Cooling TowerSiLan Subramaniam100% (1)
- Lecture 7: Isothermal Flash CalculationsDocument19 pagesLecture 7: Isothermal Flash CalculationsAREEJ JAVEDNo ratings yet
- BODR For HVACDocument3 pagesBODR For HVACMo EmadNo ratings yet
- Chapter 12 - Heat ExchangerDocument21 pagesChapter 12 - Heat ExchangerBảo Tín TrầnNo ratings yet
- Thermochemistry IB QuestionsDocument4 pagesThermochemistry IB QuestionsArmstrong NworkaNo ratings yet
- Worksheet - Melting Point DeterminationDocument4 pagesWorksheet - Melting Point DeterminationSobi SitjarNo ratings yet
- Science 7 Heat Transfer Learning Activity Sheets 6Document3 pagesScience 7 Heat Transfer Learning Activity Sheets 6fitz zamoraNo ratings yet
- BME Unit 1Document40 pagesBME Unit 1Renu PeriketiNo ratings yet
- 3 - CET-II - Solution Thermodynamics-App PDFDocument48 pages3 - CET-II - Solution Thermodynamics-App PDFshilp patelNo ratings yet
- Vapour Compression RefrigerationDocument4 pagesVapour Compression RefrigerationAhmadNo ratings yet
- ASTM-E-145-94 (Reapproved 2006) Standard Specification For Gravity-Convection and Forced-Ventilation Ovens1Document3 pagesASTM-E-145-94 (Reapproved 2006) Standard Specification For Gravity-Convection and Forced-Ventilation Ovens1Ben Aguilar100% (1)
- PCM Heat SinkDocument7 pagesPCM Heat SinkSachin BelgaonkarNo ratings yet
- Mono Splits York InverterDocument4 pagesMono Splits York Inverterbride junior tchuensuNo ratings yet